Polymerization Techniques and Reaction Mixtures
Info: 8059 words (32 pages) Dissertation
Published: 11th Dec 2019
Tagged: Chemistry
1. Polymerization techniques
- Controlled radical polymerizations
Polymeric materials have always been pivotal to man’s way of life in one form or another. From early times, natural polymers were exploited for clothing, shelter, tools, even decorations and toys. With progress in the field of polymer chemistry and the evolving needs of society for improved materials, natural materials have been steadily replaced by synthetic polymers since they became commercially available, owing to the discovery of free radical polymerization in the early 20th century.
Polymeric hybrid materials have long been prepared to modify the properties and performance of a whole host of materials from biopolymers such as cellulose to inorganic materials like silica. Hybrid materials occupy a unique position in the development of new materials with properties not accessible by any other known single material. The extent to which material properties can be altered by the modification of chemical functions, structure and blending allows the design of novel polymer hybrid materials to fulfill new and highly specific roles.
One such tool that has greatly expanded and revolutionized the frontier between polymer chemistry and materials science is the development of controlled radical polymerization techniques over the past 20 years. Since its conception, controlled/“living” radical polymerization processes have resulted in the preparation of a plethora of well-defined functional polymers with complex architectures that were previously inaccessible via conventional living ionic polymerization. Since then, the incorporation of polymer coatings to confer interesting properties to a variety of materials in a controlled and facile way has seen an explosion in research efforts towards the preparation of advanced materials. As such, controlled/“living” radical polymerization techniques have arisen as highly versatile and ubiquitous tools for material design, an exceptional step forward.
The progress of controlled radical polymerizations (CRP) technique has contributed significantly to the development of numerous polymer synthesis methodologies. CRP, amongst the other living polymerization techniques allows specific control of polymer structures, eliminating the need of highly demanding experimental conditions (Szwarc, 1956).
Radical polymerization mainly involves three main steps: initiation, propagation, and termination (Scheme 1) (Colombani, 1997). The initiation process is characterized by the generation of a reactive radical which then reacts with a monomer. Propagation involves the growth of polymer chains by monomer addition to the end chain radical. The concluding step of termination proceeds with a combination of coupling and disproportionation of two end chain radicals to produce inactive polymers. Furthermore, the chain transfer reactions are responsible for transfer the active radical between polymer chains or another species within the polymerization system. A characteristic free radical polymerization involves very short lived propagating radicals which readily terminate as the termination rate constants are much higher than the propagation rate constant (Colombani, 1997, Odian, 2004). In addition, initiation is usually slower than propagation, which suggests that as the polymerization proceeds, some chains have grown significantly while others are still initiating (Colombani, 1997). Moreover, chain transfer reactions can also hinder control by moving radicals between or even within polymer chains.
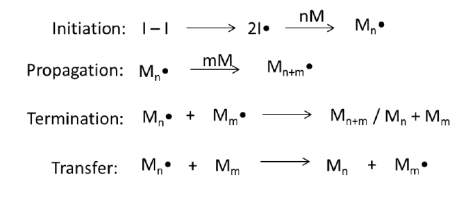
Scheme 1 A schematic representation depicting the initiation, propagation, termination and transfer. I represent the initiator and M represents the monomer (Colombani, 1997).
Otsu in the 1980s introduced the notion of CRP through his studies involving iniferters (Otsu and Yoshida, 1982, Otsu et al., 1982). The compounds capable to initiate, transfer, and terminate a radical polymerizationwere called iniferters. Nitroxide-mediated polymerization (NMP) (Hawker et al., 2001), single-electron transfer living radical polymerization (SET-LRP) (Rosen and Percec, 2009, Percec and Barboiu, 1995), atom transfer radical polymerization (ATRP) (Matyjaszewski and Spanswick, 2005, Kato et al., 1995, Wang and Matyjaszewski, 1995, Matyjaszewski et al., 1997), and reversible addition-fragmentation chain transfer (RAFT) polymerization are the main CRO techniques.
- Nitroxide-mediated polymerization (NMP)
Nitroxide- mediated polymerization (NMP) is characterized by an equilibrium state between dormant polymer chains and active radical chain ends, where nitroxide serves as the mediating or persistent radical (Scheme 1.2). As this equilibrium lies in favor of dormant species, the concentration of propagating radicals should be low and termination therefore can be curtailed (Scheme 2) (Hawker et al., 2001, Hawker, 1994).
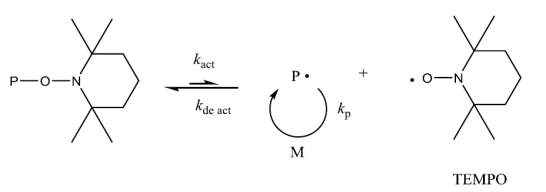
Scheme 2 Schematic mechanism of NMP (Roy et al., 2009, Braunecker et al., 2009).
- Atom transfer radical polymerization (ATRP)
In 1995, Atomic transfer radical polymerization (ATRP) was individually reported by two groups Sawamoto (Kato et al., 1995) and Matyjaszewski (Wang and Matyjaszewski, 1995). Radical generation in ATRP takes place by an organic halide undergoing a reversible redox process which is catalyzed by a transition metal compound. As this equilibrium lies in favor of the dormant species (left), the amount of radicals should be low and thus termination of living polymers can be minimized.
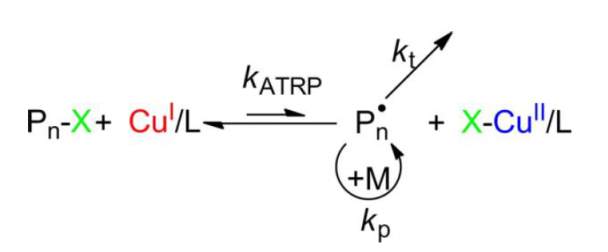
Scheme 3 Mechanism for ATRP (Pn represents polymer chain; X represents halide; L represents ligand; M represents monomer) (Zhang et al., 2013, Wang et al., 2012).
ATRP is a robust technique which is applicable to a range of monomers and is tolerant of many functional groups. In addition, the experimental conditions and operations are not strict. However, the main drawback of ATRP is due to the use of relatively large amounts of Cu (I) activator which requires removal after the polymerization
1.1.3 Reversible addition-fragmentation chain transfer polymerization (RAFT)
Reversible addition-fragmentation chain transfer polymerization (RAFT) polymerization was first reported and named in 1998 by Moad, Rizzardo, and Thang et al. in Australia (Chiefari et al., 1998). The mechanism involved in RAFT polymerization differs from ATRP and NMP. The control of RAFT polymerization is achieved by equilibrium between polymer chains led by a reversible transfer reaction using a thiocarbonyl-thio as the CTA, and not by equilibrium between a dormant species and its corresponding active radical chain end. Thus all polymer chains have equal opportunities to grow and thus achieve a controlled system. Scheme 4 depicts a RAFT polymerization mechanism, Consisting of the steps of free radical polymerization (initiation, propagation, and termination steps) and other additional steps (chain transfer and equilibration steps) (Moad et al., 2009).
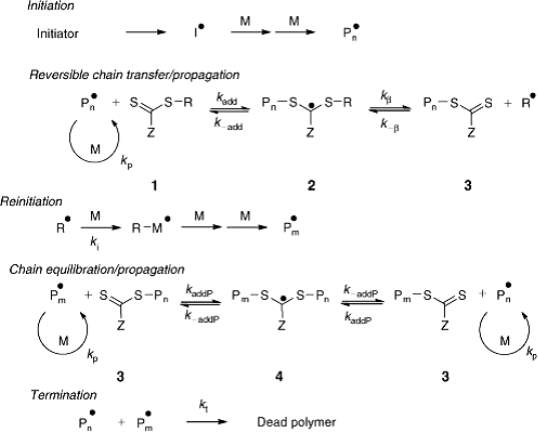
Scheme 4 Proposed general mechanism of RAFT polymerization (Moad et al., 2009).
More specifically, radical polymer species (Pn•) is formed when radical initiators decompose and then react with monomers. This growing chain gets linked to the reactive C=S bond of the CTA with the following outcomes: (1) to generate a radical intermediate, (2) This intermediate can undergo a reversible fragmentation reaction either toward starting species (Pn• and 1) or to release the R group from the CTA (R•) and a macro-CTA, (3) The R group then re-initiates and reacts with monomers to form a new growing chain (Pm•). Macro-CTA present in the reaction medium enters the main equilibrium once all the initial CTA has been depleted. This equilibrium plays a critical role in the process of RAFT polymerization as a rapid exchange between active radical chain ends and dormant ends (thiocarbonyl-thio capped) enables all polymer chains to have equal probability to grow. This ensures the production of polymers with narrow molecular weight distributions. The last step involves termination by either combination or disproportionation which is reduced in RAFT polymerization because of the presence of a CTA with high transfer efficiency, high CTA to initiator ratio and the low concentration of radical initiator used.
- Heterogeneous and dispersion polymerization
Two immiscible phases are present in heterogeneous polymerization reaction mixtures and are frequently used for synthesis of well-defined particles. The four main types of heterogeneous polymerizations usually categorized based on the solubility of the monomers, resulting polymers and initiators are: precipitation, suspension, emulsion and dispersion (Arshady, 1992). Precipitation polymerizations begin as homogeneous polymerizations where both monomers and initiators are soluble in the reaction mixture, but gets converted to heterogeneous polymerizations in the due course as the resultant polymer is insoluble in the reaction medium. The final polymers so obtained are in powder or granular form (Odian, 2004). In suspension polymerizations both the monomers and initiators are insoluble in the reaction media but are stabilized by the presence of suspension stabilizers. The initiation and polymerization occur within the droplets of monomer thereby producing insoluble polymers in the form of polymer particles (Odian, 2004, Arshady, 1992, Hohenstein and Mark, 1946). Emulsion polymerizations have soluble initiators, but the monomers and resultant polymers are insoluble in the media which are stabilized by surfactants. The initiation of emulsion polymerizations occurs in the continuous medium and polymerization take place in the micelles formed by surfactants rather than monomer droplets. Polymer particles are obtained as a result of emulsion polymerization (Arshady, 1992, Chern, 2006).
Dispersion polymerizations have significant similarity with precipitation polymerizations and also start as homogeneous polymerizations characterized by soluble monomers and initiators. The resultant insoluble polymers/growing chains in dispersion polymerizations are stabilized by additive stabilizers which are which are usually absent in precipitation polymerizations (Almog et al., 1982). Initiation of Dispersion polymerizations initiates in solution and the pre formed polymers remain in the solution until a critical molecular weight is reached. Coagulation of polymers thus forming unstable particles takes place followed by their further coagulation to form stabilized particles surrounded by stabilizers to avoid precipitation at the critical point. Polymerization further proceeds in the stabilized polymer particles which absorb monomers from the continuous phase (Odian, 2004). Dispersion polymerizations allows the synthesis of particles with a range of morphologies (Blanazs et al., 2011, Sun et al., 2012).
2. Polymerization-induced self-assembly (PISA)
Polymerization-induced self-assembly (PISA) have gained considerable attention recently due to the ease of simultaneous synthesis of block copolymers and the preparation of well-defined self-assemblies, where preparation of block copolymers is achieved in a selective solvent using soluble macro-initiator or macromolecular chain transfer agent (macro-CTA) and self-assembly is formed simultaneously during the polymerization as the growing block is insoluble in this selective solvent (Figure 1) (Sun et al., 2012).
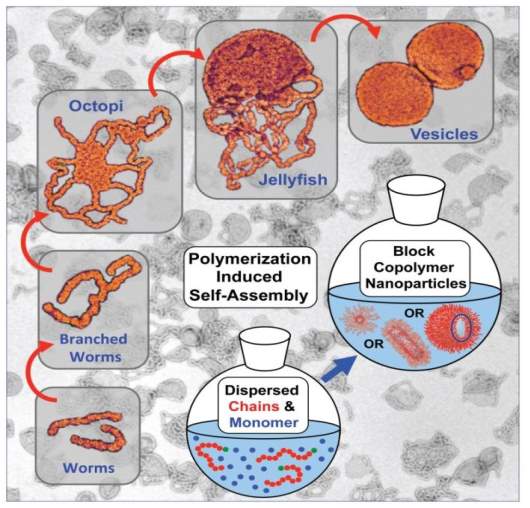
Figure 1 A diagrammatic representation of polymerization-induced self-assembly (PISA) (Blanazs et al., 2011).
Nanoparticles with various morphologies such as micelles, worm-like/rod-like and spherical vesicles are made using a polymerization-induced self-assembly (PISA) approach. The enormous advantage of this approach allows the design of systematic morphologies by simply varying the polymerization conditions (e.g., ratio of monomer to macro-CTA). Self-assemblies with controlled sizes and morphologies are formed during the polymerization process without the requirement of any further assembly and purification steps. Controlled radical polymerization techniques such as NMP, ATRP, and RAFT polymerization also find broad applications in this process (Charleux et al., 2012).
Many factors affect the resultant polymers, morphologies and morphology transitions of PISA. In general, the factors affecting solution self-assemblies prepared by post-polymerization processes have considerable effect in the process of PISA. Additionally, other factors affecting the results of PISA mainly include the nature of the macro-CTA (e.g., length, composition) (Fielding et al., 2013, Semsarilar et al., 2012), monomers (Sun et al., 2012), core-forming polymer (e.g., LCST) (Figg et al., 2015), and solvent (Sun et al., 2012), total solid content (Sugihara et al., 2011b), feed ratio of polymerization components (Sugihara et al., 2011b), cross linker (Sugihara et al., 2011a), and the addition of monomers (Zhang et al., 2014) amongst others.
Recently, studies focused on delivery of specialized agents/ biomolecules into the biological system i systems have gained considerable pace. In a recent study, encapsulation of guest molecule through the PISA process was investigated by Davis and coworkers (Karagoz et al., 2014). Encapsulation of Nile Red with high efficiency during PISA process, without disturbing the resultant morphology or kinetics of PISA system was achieved in their study (Karagoz et al., 2014). This study established that PISA has a significant potential for preparation of delivery vehicles in the medical or agricultural fields.
3. Biocompatible polymers and their biomedical applications
The primary quest which ignited the development of polymers was the search for biodegradable suture materials. A large molecule composed of many smaller units called monomers and bonded together is known as polymers. A biomaterial is defined as any natural or synthetic material engineered for the purpose of medical treatment by interacting with biological systems (Williams, 2009). These engineered materials must exhibit biocompatibility, meaning that the function carried out by them must be with an appropriate host response (Williams). The ever-growing need of the biomedical community, have necessitated the extensive study and research of materials composed of everything from metals and ceramics to glasses and polymers. The substantial potential applications of polymers in biomedical field are due to their flexibility in chemistry thereby enabling facile synthesis of materials with diverse physical and mechanical properties. Amongst these, the biodegradable polymers are the most promising candidates as the biocompatible nature of these biomaterials enables them to be broken down and excreted or resorbed without removal or surgical revision.
Extensive research exploring the biomedical applications of synthetic degradable polymers started in the 1960s and is a relatively new area of study (Epstein and Schmitt, 1975, Williams, 2009). Since then these materials have proven to be useful and successful for long-term drug delivery applications. The material of choice in these applications are biodegradable polymers due to their degradable nature in the biological system into inert and biocompatible molecules. Incorporation of drugs in biodegradable polymers enables dosage formations capable of drug release over a prolong length of time and a variety of shapes and sizes can be achieved. It also eliminates the requirement of surgical procedures after completion of dosage regime, as the remaining polymer degrade and gets cleared by the body systems.
Polymeric nanoparticles are comprised of a wide range of synthetic, natural and pseudo- synthetic polymers (Naahidi et al., 2013). Their architecture, composition, stability, solubility and to provide protection to the drug, controlled release of the encapsulated drugs and their capacity to be target specific makes them excellent carriers of drugs and other biomolecules(Qiu and Bae, 2006). Another advantage of these nanoparticles is their ability to overcome poor solubility of some drugs and enhanced their bio-distribution (Li et al., 2009). Various shapes and geometry such as nanospheres or nanocapsules can be readily attained by these particles (Reis et al., 2006). Hydrophilic and hydrophobic drugs can be attached to the nanoparticle’s surface or may be encapsulated inside their core.
The most commonly used biodegradable polymers are poly (lactic-co-glycolic acid) PLGA, polylactic acid (PLA), poly-ε-caprolactone (PCL), poly-alkyl-cyanoacrylates (PAC) and chitosan (Estanqueiro et al., 2015).
PLA is a biocompatible and biodegradable polymer that is easily eliminated from the organism once it suffers scission into monomeric units of lactic acid (Estanqueiro et al., 2015). Xing et al. reported that oridonin PLA entrapped nanoparticles enhanced the solubility and extend the blood circulation time of this diterpenoid that induces cytotoxicity to a wide variety of cancer cells (Xing et al., 2007, Wang et al., 2013).
PLC is particularly interesting for the preparation of long-term implantable devices thanks to its longer degradation time, comparing to polylactide (Estanqueiro et al., 2015). Several anticancer drug molecules used for cancer treatment had been studied by incorporation into PCL aiming to improve the therapeutic index of these molecules (Shenoy and Amiji, 2005, Kim and Lee, 2001).
PAC is a biodegradable and biocompatible polymer which can be prepared by several methods such as emulsion polymerization, interfacial polymerization and nanoprecipitation (Kumari et al., 2010). The encapsulation of various antibacterials (ampicillin), anti-inflammatory (indomethacin) and anticancer drugs (doxorubicin and ftorafur) has been used for delivery using PAC nanoparticles which have shown the polymer to be a strong candidate for future investigations as a drug release vehicle (Kumari et al., 2010).
Poly (lactic-co-glycolic acid) (PLGA) is a co-polymer widely used for drug delivery applications. The hydrolysis products of PLGA are lactic acid and glycolic acid, which are metabolites, thus being considered a biodegradable and biocompatible polymer and approved by FDA (Ma and Mumper, 2013). PLGA NPs have been described as efficient nanocarriers for different anti-cancer agents such as paclitaxel (Fonseca et al., 2002), 9-nitrocamptothecin (Derakhshandeh et al., 2007), cisplatin (Avgoustakis et al., 2002), and others, and even for several other drugs as haloperidol (Budhian et al., 2005), estradiol (Mittal et al., 2007), etc.
Naturally-derived polymers include bio-polymers like chitosan, hyaluronic acid, cellulose, fibrin, collagen, and gelatin. These polymers offer good biocompatibility that may positively support cell adhesion and function, and most exhibit biodegradable linkages that further lower toxicity. Derived from the crustacean, natural biopolymer chitin, chitosan is a modified carbohydrate polymer that has successfully encapsulated several molecules for in vivo purposes (Kumari et al., 2010). Chitosan nanoparticles have served as matrixes of a range of drugs from antihormonal (glycyrrhizin) to insulin, where the intestinal absorption following oral administration was enhanced; chitosan nanoparticles have also showed great results regarding drugs delivery to the ocular surface (Kumari et al., 2010). De Campos et al. showed that chitosan NPs loaded with cyclosporin A can contact directly with corneal and conjunctiva surfaces, where the delivery is more effective and the inner ocular structures are not compromised, as well as, the systemic drug exposure is reduced (De Campos et al., 2001).
4. Antibody conjugation to Polymeric particles
The surface modification of nanoscale drug carriers involves chemically coupling various molecules on the outer surface of a polymeric nanoparticle or liposome. The surface modification serves various goals: i) covalently coupling targeting ligands (e.g., antibodies, proteins or peptides ) for active tissue/cell targeting; ii) labeling drug carriers (e.g., with fluorescent molecules) for in vitro/vivo imaging or diagnosis; iii) coupling drug molecules for delivery purposes, or iv) coupling a combination of the above mentioned molecules simultaneously for multiple functionalization.
Over the past decade, surface modifications have offered drug carriers additional functionalization and extended versatility (Peer et al., 2007, Allen, 2002). Different moieties such as peptides, proteins, low molecular weight ligands, polyunsaturated fatty acids, polysaccharides, DNA, and so forth can be conjugated to the nanoparticles thereby enhancing the properties of nanoparticles thus widening their applications in various fields. The antibody conjugated nanoparticles show improved cellular and intracellular stability thus making its usage advantageous.
Antibodies are glycoproteins belonging to the immunoglobulin super family [41]. An antibody molecule is a homo-dimer composed of two identical heavy and two identical light chains. Both the heavy chains are linked together by two disulphide bridges at the hinge region. Likewise, each light chain is linked to its respective heavy chain by a disulphide bridge. Each antibody molecule consists of a constant domain (Fc) and two antigen-binding domains (Fab) which are hypervariable and responsible for antigen recognition and specificity. Each chain has carboxyl (-COOH) and amino (-NH2) groups, which are contributed by terminal and internal amino acids. A typical antibody (IgG2) with all the available functional groups is illustrated in Figure 2. The antibody along with its isotypes is an important component of the immune system; present either in soluble form or as membrane-bound surface receptors on B-cells. A monoclonal antibody is highly specific to a single defined region or epitope on an antigen and binds only to that region; whereas, polyclonal antibody consists of a blend of antibodies which can recognize a wide range of antigens or multiple epitopes on a single antigen.
Covalent immobilization of antibody is preferred over physisorption as stable and high protein coverage is achieved with least susceptibility to leaching or protein exchange. This technique requires prior activation of antibody molecules (by linkers) which then react chemically with the active functional groups present on the substrate via free reactive groups of the antibody such as amine or carboxyl groups.
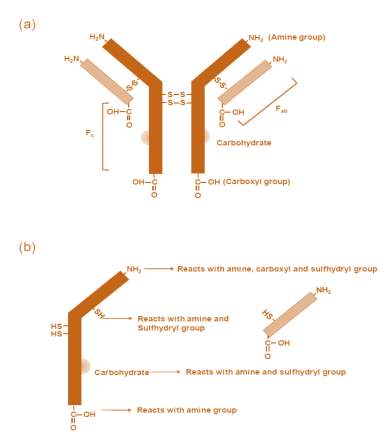
Figure 2 A typical IgG antibody with the available functional groups.
For the preparation of targeted nanoscale drug carriers, chemical modifications are carried out, in almost all cases, after the formation of nanoscale carriers and/or the incorporation of drugs, using functional groups that are accessible to targeting ligands. To maintain the functions of drug carriers and the bioactivity of coupled targeting ligands/incorporated drugs, a desirable conjugation chemistry should be simple, efficient, site-selective, and environmentally friendly (preferably in aqueous environments), occur under mild conditions (under 50°C and pH values between 6 and 8), and produce a stable and nontoxic chemical bond. The different conjugation strategies involve Chemistry between Maleimide and Thiol, Chemistry between Carboxylic Acid and Amine, Disulfide Exchange, Chemistry between p-Nitrophenylcarbonyl and Amine, Hydrazide coupling, Chemistry between Vinylsulfone and Thiol, Avidin-Biotin Interaction, etc.
5. Fluorescence Spectroscopy
Fluorescence spectroscopy commonly referred to as fluorometry or spectrofluorometry is a kind of electromagnetic spectroscopy used for analysis of fluorescence from a sample. It has been extensively used as an analytical tool by scientists for many years.
Different molecules have different states referred to as energy levels. The species which is being examined usually has a ground electronic state (a low energy state), and an excited electronic state exhibiting higher energy. Various other vibration states are present within each of these electronic states (Montalti et al., 2006).
In a typical fluorescence analysis, the species being examined is first excited from its ground electronic state to one of the various vibrational states in the excited electronic state by a photon. The excited molecule loses its vibrational energy due to with other molecules and eventually reaches the lowest vibrational state of the excited electronic state. This process is often depicted with a Jablonski diagram (Montalti et al., 2006).
A photon is released when the molecule drops down to one of the various vibrational levels of the ground electronic state (Montalti et al., 2006). As molecules may drop down into any of several vibrational levels in the ground state, the emitted photons will have different energies, and thus frequencies. Therefore, analysis of the different frequencies of emitted light in fluorescent spectroscopy, along with their relative intensities, the structure of the different vibrational levels can be determined.
During fluorescence spectroscopic analysis, molecules in a sample undergo electronic transitions based on their bonding structure. Certain bonding types in molecules create higher probability of fluorescence emission. Aromatic compounds are the most likely to fluoresce because of their delocalized pi bonding. Strongly localized sigma bonding structure does not enable fluorescence emission.
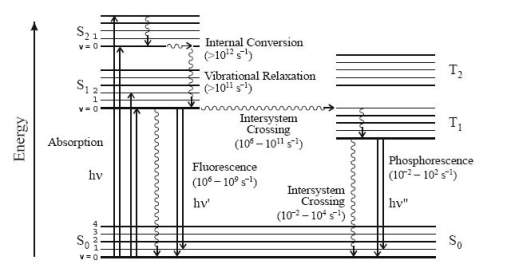
Figure 3 Jablonski diagram showing electron transition processes. (Montalti et al., 2006).
6. Cellular imaging
It has been repeatedly demonstrated that many different types of nanoparticles will enter cells, but it is often unclear by which route this uptake occurs. Cells have the ability to internalize a range of substances, from small, single molecules through to larger particles such as bacteria. This uptake can occur through a number of different pathways which are all important and work together in order to maintain life, allow cell signaling and migration and protect the cell from infectious agents. Some of these routes of uptake are also exploited by nanoparticles. Investigations into the internalization of nanoparticles into cells are important because of the human health effects of exposure to nanoparticles of various different shapes, sizes and compositions through, for example, air pollution and cosmetics (Kemp et al., 2008, Zhao et al., 2011). Nanoparticles are also being used in an increasing range of applications including diagnostics, measurement of intracellular analytes such as calcium and oxygen, imaging, phototherapy and gene and small molecule drug delivery (Cheng et al., 2006, Chithrani et al., 2006, O’Riordan et al., 2007). Nanoparticles have unique properties due to their size and they often behave very differently to a bulk amount of the same material (Labhasetwar, 2005). Their small size, ranging from a few nanometres up to several hundred nanometres, enables them to be present inside cells with minimal disturbance of the cell itself. Nanoparticles therefore have great potential for a variety of biological applications due to their ability to enter cells . (Zhao et al., 2011).
Different pathways of cellular uptake enable cells to maintain life via the internalization of molecules required for many cellular processes including development, neurotransmission, cell-cell communication and signaling. Small molecules and ions do not require a carrier system; they can diffuse or cross the membrane using protein pumps and channels in the bilayer. Larger molecules, greater than a few nanometres in size in general do not have this ability, therefore they need to be transported into the cell in a different way. This transport can occur via a number of different endocytic pathways including phagocytosis, clathrin mediated endocytosis, caveolae mediated endocytosis and macropinocytosis. These pathways are often distinct in the molecules they transport into the cell and they are highly regulated by a number of proteins. However, they have the potential to be upregulated depending on the cellular environment, inhibition of one of the other endocytic pathways, and the needs of the cell13. Phagocytosis, macropinocytosis, clathrin and caveolae mediated endocytosis have all been implicated in the energy dependent uptake of nanoparticles into cells (Zhao et al., 2011).
Visual information about the interaction of nanoparticles with cell membranes on the nanoscale has been difficult to obtain in the past. This is due to limitations in the resolution of imaging techniques, and the complex nature and relatively rapid speed of the uptake processes. Imaging of the intracellular localization of nanoparticles following uptake has generally been performed using fluorescence microscopy, which has a limited maximum resolution of approximately 200nm. However, imaging technology has improved and it is now possible to obtain some of this information using a combination of advanced imaging techniques such as Atomic Force Microscopy (AFM), which can be used to obtain nanoscale images of the interaction of nanoparticles with small regions of model cell membranes and increasingly, cells.
References
ALLEN, T. M. 2002. Ligand-targeted therapeutics in anticancer therapy. Nature reviews. Cancer, 2, 750.
ALMOG, Y., REICH, S. & LEVY, M. 1982. Monodisperse polymeric spheres in the micron size range by a single step process. Polymer International, 14, 131-136.
ARSHADY, R. 1992. Suspension, emulsion, and dispersion polymerization: A methodological survey. Colloid & Polymer Science, 270, 717-732.
AVGOUSTAKIS, K., BELETSI, A., PANAGI, Z., KLEPETSANIS, P., KARYDAS, A. & ITHAKISSIOS, D. 2002. PLGA–mPEG nanoparticles of cisplatin: in vitro nanoparticle degradation, in vitro drug release and in vivo drug residence in blood properties. Journal of Controlled Release, 79, 123-135.
BLANAZS, A., MADSEN, J., BATTAGLIA, G., RYAN, A. J. & ARMES, S. P. 2011. Mechanistic insights for block copolymer morphologies: how do worms form vesicles? Journal of the American Chemical Society, 133, 16581-16587.
BRAUNECKER, W. A., TSAREVSKY, N. V., GENNARO, A. & MATYJASZEWSKI, K. 2009. Thermodynamic components of the atom transfer radical polymerization equilibrium: quantifying solvent effects. Macromolecules, 42, 6348-6360.
BUDHIAN, A., SIEGEL, S. J. & WINEY, K. I. 2005. Production of haloperidol-loaded PLGA nanoparticles for extended controlled drug release of haloperidol. Journal of microencapsulation, 22, 773-785.
CHARLEUX, B., DELAITTRE, G., RIEGER, J. & D’AGOSTO, F. 2012. Polymerization-induced self-assembly: from soluble macromolecules to block copolymer nano-objects in one step. Macromolecules, 45, 6753-6765.
CHENG, M. M.-C., CUDA, G., BUNIMOVICH, Y. L., GASPARI, M., HEATH, J. R., HILL, H. D., MIRKIN, C. A., NIJDAM, A. J., TERRACCIANO, R. & THUNDAT, T. 2006. Nanotechnologies for biomolecular detection and medical diagnostics. Current opinion in chemical biology, 10, 11-19.
CHERN, C. 2006. Emulsion polymerization mechanisms and kinetics. Progress in polymer science, 31, 443-486.
CHIEFARI, J., CHONG, Y., ERCOLE, F., KRSTINA, J., JEFFERY, J., LE, T. P., MAYADUNNE, R. T., MEIJS, G. F., MOAD, C. L. & MOAD, G. 1998. Living free-radical polymerization by reversible addition− fragmentation chain transfer: the RAFT process. Macromolecules, 31, 5559-5562.
CHITHRANI, B. D., GHAZANI, A. A. & CHAN, W. C. 2006. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano lett, 6, 662-668.
COLOMBANI, D. 1997. Chain-growth control in free radical polymerization. Progress in polymer science, 22, 1649-1720.
DE CAMPOS, A. M., SÁNCHEZ, A. & ALONSO, M. A. J. 2001. Chitosan nanoparticles: a new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. International journal of pharmaceutics, 224, 159-168.
DERAKHSHANDEH, K., ERFAN, M. & DADASHZADEH, S. 2007. Encapsulation of 9-nitrocamptothecin, a novel anticancer drug, in biodegradable nanoparticles: factorial design, characterization and release kinetics. European journal of pharmaceutics and biopharmaceutics, 66, 34-41.
EPSTEIN, M. & SCHMITT, E. E. 1975. Reducing capillarity of polyglycolic acid sutures. Google Patents.
ESTANQUEIRO, M., AMARAL, M. H., CONCEIÇÃO, J. & LOBO, J. M. S. 2015. Nanotechnological carriers for cancer chemotherapy: the state of the art. Colloids and surfaces B: Biointerfaces, 126, 631-648.
FIELDING, L. A., DERRY, M. J., LADMIRAL, V., ROSSELGONG, J., RODRIGUES, A. M., RATCLIFFE, L. P., SUGIHARA, S. & ARMES, S. P. 2013. RAFT dispersion polymerization in non-polar solvents: facile production of block copolymer spheres, worms and vesicles in n-alkanes. Chemical Science, 4, 2081-2087.
FIGG, C. A., SIMULA, A., GEBRE, K. A., TUCKER, B. S., HADDLETON, D. M. & SUMERLIN, B. S. 2015. Polymerization-induced thermal self-assembly (PITSA). Chemical Science, 6, 1230-1236.
FONSECA, C., SIMOES, S. & GASPAR, R. 2002. Paclitaxel-loaded PLGA nanoparticles: preparation, physicochemical characterization and in vitro anti-tumoral activity. Journal of Controlled Release, 83, 273-286.
HAWKER, C. J. 1994. Molecular weight control by a” living” free-radical polymerization process. Journal of the American Chemical Society, 116, 11185-11186.
HAWKER, C. J., BOSMAN, A. W. & HARTH, E. 2001. New polymer synthesis by nitroxide mediated living radical polymerizations. Chemical reviews, 101, 3661-3688.
HOHENSTEIN, W. & MARK, H. 1946. Polymerization of olefins and diolefins in suspension and emulsion. Part I. Journal of Polymer Science Part A: Polymer Chemistry, 1, 127-145.
KARAGOZ, B., BOYER, C. & DAVIS, T. P. 2014. Simultaneous Polymerization‐Induced Self‐Assembly (PISA) and Guest Molecule Encapsulation. Macromolecular rapid communications, 35, 417-421.
KATO, M., KAMIGAITO, M., SAWAMOTO, M. & HIGASHIMURA, T. 1995. Polymerization of methyl methacrylate with the carbon tetrachloride/dichlorotris-(triphenylphosphine) ruthenium (II)/methylaluminum bis (2, 6-di-tert-butylphenoxide) initiating system: possibility of living radical polymerization. Macromolecules, 28, 1721-1723.
KEMP, S. J., THORLEY, A. J., GORELIK, J., SECKL, M. J., O’HARE, M. J., ARCARO, A., KORCHEV, Y., GOLDSTRAW, P. & TETLEY, T. D. 2008. Immortalization of human alveolar epithelial cells to investigate nanoparticle uptake. American journal of respiratory cell and molecular biology, 39, 591-597.
KIM, S. Y. & LEE, Y. M. 2001. Taxol-loaded block copolymer nanospheres composed of methoxy poly (ethylene glycol) and poly (ε-caprolactone) as novel anticancer drug carriers. Biomaterials, 22, 1697-1704.
KUMARI, A., YADAV, S. K. & YADAV, S. C. 2010. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids and Surfaces B: Biointerfaces, 75, 1-18.
LABHASETWAR, V. 2005. Nanotechnology for drug and gene therapy: the importance of understanding molecular mechanisms of delivery. Current opinion in biotechnology, 16, 674-680.
LI, X., YANG, Z., YANG, K., ZHOU, Y., CHEN, X., ZHANG, Y., WANG, F., LIU, Y. & REN, L. 2009. Self-assembled polymeric micellar nanoparticles as nanocarriers for poorly soluble anticancer drug ethaselen. Nanoscale research letters, 4, 1502.
MA, P. & MUMPER, R. J. 2013. Paclitaxel nano-delivery systems: a comprehensive review. Journal of nanomedicine & nanotechnology, 4, 1000164.
MATYJASZEWSKI, K., PATTEN, T. E. & XIA, J. 1997. Controlled/“living” radical polymerization. Kinetics of the homogeneous atom transfer radical polymerization of styrene. Journal of the American Chemical Society, 119, 674-680.
MATYJASZEWSKI, K. & SPANSWICK, J. 2005. Controlled/living radical polymerization. Materials Today, 8, 26-33.
MITTAL, G., SAHANA, D., BHARDWAJ, V. & KUMAR, M. R. 2007. Estradiol loaded PLGA nanoparticles for oral administration: effect of polymer molecular weight and copolymer composition on release behavior in vitro and in vivo. Journal of Controlled Release, 119, 77-85.
MOAD, G., RIZZARDO, E. & THANG, S. H. 2009. Living radical polymerization by the RAFT process–a second update. Australian journal of chemistry, 62, 1402-1472.
MONTALTI, M., CREDI, A., PRODI, L. & GANDOLFI, M. T. 2006. Handbook of photochemistry, CRC press.
NAAHIDI, S., JAFARI, M., EDALAT, F., RAYMOND, K., KHADEMHOSSEINI, A. & CHEN, P. 2013. Biocompatibility of engineered nanoparticles for drug delivery. Journal of controlled release, 166, 182-194.
O’RIORDAN, T. C., FITZGERALD, K., PONOMAREV, G. V., MACKRILL, J., HYNES, J., TAYLOR, C. & PAPKOVSKY, D. B. 2007. Sensing intracellular oxygen using near-infrared phosphorescent probes and live-cell fluorescence imaging. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 292, R1613-R1620.
ODIAN, G. 2004. Principles of polymerization, John Wiley & Sons.
OTSU, T. & YOSHIDA, M. 1982. Role of initiator‐transfer agent‐terminator (iniferter) in radical polymerizations: Polymer design by organic disulfides as iniferters. Macromolecular Rapid Communications, 3, 127-132.
OTSU, T., YOSHIDA, M. & TAZAKI, T. 1982. A model for living radical polymerization. Macromolecular Rapid Communications, 3, 133-140.
PEER, D., KARP, J. M., HONG, S., FAROKHZAD, O. C., MARGALIT, R. & LANGER, R. 2007. Nanocarriers as an emerging platform for cancer therapy. Nature nanotechnology, 2, 751-760.
PERCEC, V. & BARBOIU, B. 1995. ” Living” radical polymerization of styrene initiated by arenesulfonyl chlorides and CuI (bpy) nCl. Macromolecules, 28, 7970-7972.
QIU, L. Y. & BAE, Y. H. 2006. Polymer architecture and drug delivery. Pharmaceutical research, 23, 1-30.
REIS, C. P., NEUFELD, R. J., RIBEIRO, A. J. & VEIGA, F. 2006. Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomedicine: Nanotechnology, Biology and Medicine, 2, 8-21.
ROSEN, B. M. & PERCEC, V. 2009. Single-electron transfer and single-electron transfer degenerative chain transfer living radical polymerization. Chemical reviews, 109, 5069-5119.
ROY, D., SEMSARILAR, M., GUTHRIE, J. T. & PERRIER, S. 2009. Cellulose modification by polymer grafting: a review. Chemical Society Reviews, 38, 2046-2064.
SEMSARILAR, M., JONES, E. R., BLANAZS, A. & ARMES, S. P. 2012. Efficient Synthesis of Sterically‐Stabilized Nano‐Objects via RAFT Dispersion Polymerization of Benzyl Methacrylate in Alcoholic Media. Advanced Materials, 24, 3378-3382.
SHENOY, D. B. & AMIJI, M. M. 2005. Poly (ethylene oxide)-modified poly (ɛ-caprolactone) nanoparticles for targeted delivery of tamoxifen in breast cancer. International journal of pharmaceutics, 293, 261-270.
SUGIHARA, S., ARMES, S. P., BLANAZS, A. & LEWIS, A. L. 2011a. Non-spherical morphologies from cross-linked biomimetic diblock copolymers using RAFT aqueous dispersion polymerization. Soft Matter, 7, 10787-10793.
SUGIHARA, S., BLANAZS, A., ARMES, S. P., RYAN, A. J. & LEWIS, A. L. 2011b. Aqueous dispersion polymerization: a new paradigm for in situ block copolymer self-assembly in concentrated solution. Journal of the American Chemical Society, 133, 15707-15713.
SUN, J.-T., HONG, C.-Y. & PAN, C.-Y. 2012. Formation of the block copolymer aggregates via polymerization-induced self-assembly and reorganization. Soft Matter, 8, 7753-7767.
SZWARC, M. 1956. ‘Living’polymers.
WANG, J.-S. & MATYJASZEWSKI, K. 1995. Controlled/” living” radical polymerization. Atom transfer radical polymerization in the presence of transition-metal complexes. Journal of the American Chemical Society, 117, 5614-5615.
WANG, S., ZHONG, Z., WAN, J., TAN, W., WU, G., CHEN, M. & WANG, Y. 2013. Oridonin induces apoptosis, inhibits migration and invasion on highly-metastatic human breast cancer cells. The American journal of Chinese medicine, 41, 177-196.
WANG, Y., KWAK, Y., BUBACK, J., BUBACK, M. & MATYJASZEWSKI, K. 2012. Determination of ATRP equilibrium constants under polymerization conditions. ACS Macro Letters, 1, 1367-1370.
WILLIAMS, D. The Williams Dictionary of Biomaterials Liverpool, UK: Liverpool. University Press.–1999.–42 p.
WILLIAMS, D. F. 2009. On the nature of biomaterials. Biomaterials, 30, 5897-909.
XING, J., ZHANG, D. & TAN, T. 2007. Studies on the oridonin-loaded poly (D, L-lactic acid) nanoparticles in vitro and in vivo. International journal of biological macromolecules, 40, 153-158.
ZHANG, Q., WILSON, P., LI, Z., MCHALE, R., GODFREY, J., ANASTASAKI, A., WALDRON, C. & HADDLETON, D. M. 2013. Aqueous copper-mediated living polymerization: exploiting rapid disproportionation of CuBr with Me6TREN. J. Am. Chem. Soc, 135, 7355-7363.
ZHANG, W.-J., HONG, C.-Y. & PAN, C.-Y. 2014. Fabrication of spaced concentric vesicles and polymerizations in RAFT dispersion polymerization. Macromolecules, 47, 1664-1671.
ZHAO, F., ZHAO, Y., LIU, Y., CHANG, X., CHEN, C. & ZHAO, Y. 2011. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small, 7, 1322-1337.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Chemistry"
Chemistry is a science involving the study of the elements and matter at the atomic and molecular level including their composition, structure, properties, behaviour, and how they react or combine.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




