Thermoplastic Polyurethanes Chemical Evaluation
Info: 5707 words (23 pages) Dissertation
Published: 10th Dec 2019
Tagged: Chemistry
Chapter 2 Literature Review
2.1 Thermoplastics
Thermoplastic polymers consist of long, linear polymeric chains or branched chains that are held together by secondary bonds, like hydrogen bonds and weak Van der Waals forces. These materials are sensitive to heat; as such, the intermolecular forces between chains are weakened when these polymers are heated, resulting in a soft and flexible polymer [1][2]. Thermoplastic polymers become viscous and melt when they are heated at high temperatures [1]. Nonetheless, these polymers solidify again with cooling and can partially crystallise [1][2]. As a result, these materials can be moulded and remoulded via heating [2]. Good examples of thermoplastic polymers are polymethyl methacrylate, polyethylene, polystyrene polyvinyl chloride, polystyrene, nylon, polycarbonate, polyurethane and polypropylene [1].
Thermoplastic polymers can be classified depending on their crystallinity such as whether they have a semi-crystalline or an amorphous structure [1][3]. It is impossible to produce entirely crystalline plastics by moulding due to the complex physical nature of the molecular chains. On the other hand, other thermoplastics like acrylic and polystyrene are amorphous [1].Semi-crystalline thermoplastic polymers such as polyethylene, polypropylene and polyamide are opaque, and have high density, high shrinkage, high chemical resistance, and a sharp melting point. In contrast, amorphous thermoplastic polymers such as polyvinyl chloride, polystyrene and polycarbonate are transparent, and have low density, a broad softening point, low shrinkage, and low chemical resistance [1].
Polymers such as polyetheretherketone (PEEK) polyetherimide, (PEI), polyphenylene sulphide (PPS) and polyamide (PA) are extensively used as matrices to produce thermoplastic composites with extensive mechanical properties. However, the cost of producing these composites is expensive because of the high processing temperature required, resulting from the high melting point of their matrices [4][5][6]. In contrast, polypropylene (PP) represents one of the most semi-crystalline thermoplastics widely used for general applications. This can be attributed to its low cost, lightweight, low melting point and suitable mechanical properties like stiffness and strength [7]. Nevertheless, GF-PP composites exhibit far fewer mechanical properties compared to GF-PA composites. These drawbacks have pushed many researchers [8][9][10][11][12][13][14] to design and develop thermoplastic polyurethanes (TPUs) that have attracted increased attention in recent decades. This is due to TPUsʼ combination of a low melting point, lower energy cost, high processing speed with good mechanical properties, all of which make it the best candidate for use in polymeric matrices instead of PP and PA.
2.1.1 Thermoplastic Polyurethanes (TPUs)
In industry, most polymers are manufactured from as single monomer, resulting in homopolymers. In contrast, polyurethanes have more complicated chemical structures, and they usually consist of three monomers: a diisocyanate, a polyol and a chain extender (diol or diamine). Unlimited numbers of TPUs can be manufactured from these monomers with different structural physicochemical and mechanical properties, due to the degrees of freedom available when using the three monomers [2][15].
Thermoplastic polyurethanes (TPUs) are linear segmented diblock copolymers consisting of alternating soft segments (SS). The SS are separated by corresponding hard segments (HS) formed by di-urethane groups with intermolecular hydrogen bonds [16][3][17][18][19][20] (see Figure 2.1) [17]. Urethane linkages (-NH-CO-O-) are created by the reaction of diisocyanate (aromatic or aliphatic) with a long-chain diol (a polyol with high molecular weight) or with a short-chain diol (a chain extender with low molecular weight). These monomers are linked together by covalent bonds [16][3][21]. Polyurethanes with 100% HS content are known as homopolyurethanes. They have a higher number of hydrogen bonds than polyurethanes, which are composed of both soft and hard segments [17].
Soft segments (Tg below ambient temperature) are amorphous and usually consist of a sequence of long-chain polyether or polyester polyols [16][3][15][17][18]. They are flexible at low temperatures, and are resistant to weatherability and solvents [15][18]. Conversely, hard segments (Tg above ambient temperature) are usually composed of a sequence of both a diisocyanate and a chain extender. At ambient temperature, hard segments that are glassy and/or semi-crystalline are associated to form hard segment domains, acting as physical crosslinks. These hard segments also serve as filler particles within the rubbery soft segments matrix, due to their rigidity and hydrogen bonding [16][3][15][17][18]. Physical crosslinks that are achieved by hydrogen bonding and hard segment domains formation prevent the TPU chains from sliding against their neighbours and causing plastic flow. As such, the TPU can be re-melted or dissolved with physical crosslinks. Hard segments may be able to crystallise because they have large interchain attraction resulting from hydrogen bonding between the urethane groups [17]. Moreover, TPUs represent elastomeric materials that combine flexible, elastic segments with a low glass-transition temperature and rigid crystallising segments with a high melting point [22].
Soft segments are terminated with hydroxyl (OH) groups, whilst chain extenders that are generally small molecules are terminated with either OH groups or amine groups. On the other hand, diisocyanates can easily react with polyols or chain extenders to produce segmented polyurethanes. Linear polyurethanes can be produced by using three bi-functional monomers (diisocyanate, polyol and chain extender), while branched or cross-linked polyurethanes are produced by using multifunctional isocyanate, polyol and occasionally chain extender [15]. In other words, in order to achieve high molecular-weight linear TPU chains, pre-polymer and monomer units have to possess two terminal groups [16].
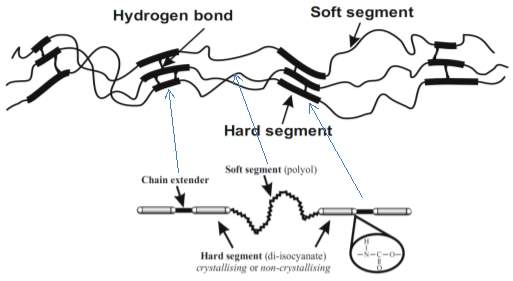
Figure 2.1: Structure of repeated hard segments and soft segments of a typical TPU [17].
The range of molecular weights of polyols usually used in the synthesis of TPU is 200-10,000 g/mol [18]. The glass-transition temperature (Tg) of the soft segments is generally between -40 and -80oC [23]. Hydrogen bonds and Vander Waalsʼ forces, such as dipole-dipole interaction and dipole-induced-dipole interaction link together the hard segments and form domains as physical crosslinks (see Figure 2.2) [13][23].
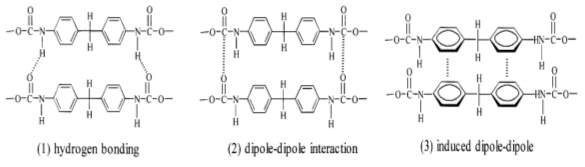
Figure 2.2: Hydrogen bonding and Vander Waalsʼ forces between polyurethane chains [13].
The ratio of the soft and hard segments, the nature of the raw materials and reaction conditions play an important role in determining the properties and structure of TPUs [21][22]. Therefore, mechanical, physical and thermal properties such as rigidity, hardness, elongation, strength, low-temperature flexibility and Tg can be changed [22].
Aromatic thermoplastic polyurethanes based on diphenylmethane diisocyanate (MDI) and toluene diisocyanate (TDI) can be used in different applications which require strength, flexibility and toughness. Alternatively, aliphatic thermoplastic polyurethanes based on 1,6-hexane diisocyanate (HDI), methylene bis (p-cyclohexyl isocyanate) (H12 MDI) and isophorone diisocyanate (IPDI) show superior optical clarity [24].
There are three main types of thermoplastic polyurethane: polyether, polyester and polycaprolactone (biodegradable polyester).
- Polyether-based TPUs show low-temperature flexibility and good acid / base resistance. Furthermore, they have excellent resistance to microbial and fungus attack and to hydrolysis. As such, they are suitable for underwater applications [16][21][24][25].
- Polyester-based TPUs provide good chemical solutions, solvent and oil resistance, as well as having good abrasion resistance, weatherability, heat resistance, UV resistance and mechanical properties [16][21][22][25].
- Polycaprolactone-based TPUs have toughness and a comparatively high resistance to hydrolysis combined with good low-temperature performance [24].
TPUs are largely used in automotive manufacturing, wires and cables, hoses and tubing, wheels and casters, film and sheets, adhesives, sealants and coatings, and medical applications like tubing catheters. TPUs can be processed by the same methods that are used to process the thermoplastics [16]. Many methods are used to process TPUs, such as blowing, extrusion, injection and compression moulding [16][26]. As a result, investments in polyurethane research will most probably increase for many years due to market needs and new inventions.
2.1.1.1 Chemistry of TPUs
Polymer-synthesis methods play an important role in the structure of the final polymer, such as the branching degree of the polymer chains and the distribution of the molecular weight. Homopolymers and copolymers can be produced by additional polymerisation. The specific kind of copolymer produced, which may include alternating, block or random, depends on the reactivity and concentration ratios of the monomers and the reaction conditions. Copolymers can be produced by condensation polymerisation [27].
Reactions of TPU synthesis include both features of addition and condensation polymerisations. The reactions between a diisocyanate and a polyol or chain extender can be classified as condensation reactions, although TPU-polymerisation reactions do not produce small molecules as eliminated materials. This is because the kinetics of TPU-polymerisation are more analogous to those of condensation than addition [27]. TPUs can be synthesised in either a one-step reaction or in two steps in order to create each phase separately [27][28].
The main chemical reaction in polyurethane synthesis is the reaction between the isocyanate and the OH group (urethane-forming reaction). The reaction between the isocyanate and the chain extender (diol or diamine) is considered another important reaction in polyurethane synthesis. When a diol chain extender is used, urethane linkage will be formed, whereas when a diamine chain extender is used, urea linkage will be created. The reaction of isocyanate with a primary amine is much stronger than its reaction with a secondary amine like N-H in urea or urethane groups [15].
One-Step (One-Shot) Method
The one-step method is considered one of the fastest and simpleest manufacturing techniques. As a result, many companies use this approach to synthesise polyurethanes which are produced by the reaction of an isocyanate (with or without chain extender) and a polyol at the same time. A solvent and a catalyst are used in this method to synthesise polyurethanes [28]. The polymer produced in this method is of a low molecular weight and random structure [20][23][27][29]. The sequence of hard segments in the polyurethane chains depends on the reactivity difference between the hydroxyl groups of the chain extender and the polyol with various isocyanate groups. So, polyurethanes produced using this method have a more random sequence [29].
Two-Step (Pre-Polymer) Method
The pre-polymer method, which is a two-step method, can be used to synthesise TPUs. The reaction of TPU synthesis can be performed in two steps. In the first step, the reaction can be carried out between large excess diisocyanate and polyol to produce an isocyanate-terminated pre-polymer with excess diisocyanate monomer [20][27][29]. The pre-polymer produced in this method has a low molecular weight [20][27]. In the second step, the pre-polymer, excess diisocyanate and chain extender can react to produce the multi-block copolymer TPUs [20][27][29] (see Figure 2.3) [20]. The two-step method is preferred, as the physical properties, structure and reactivity of the final polymer can be controlled [27]. A solvent and a catalyst are used in the synthesis of TPUs in this method [20].
The sequence in the TPU which is carried out by the pre-polymer method is more regular than that used in the one-shot method. Consequently, better packing of the hard segments can be attained by the structural regularity of this method, and it is easier to form points of physical crosslinking. As such, better mechanical properties of polymer can be obtained using a two-step method than with a one-step method. In general, solvent is used in polyurethane synthesis in laboratories to reduce the viscosity and enhance the formation of copolymers with high molecular weight. On the other hand, commercial polyurethanes are synthesised without solvent in either the one-step or the two-step method [15].
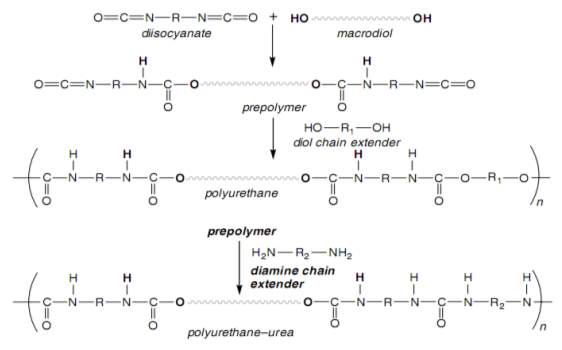
Figure 2.3: Two-step method of synthesis of thermoplastic polyurethane and polyurethane –urea [20].
2.1.1.2 Morphology and Properties of TPUs
The chain structure of SS and HS segments plays an important role in determining the properties of TPU. Changes in TPU properties connect with the molecular weight and chemical structure of SS, crystallisation of SS and HS, and the percentage of HS, as well as the type of isocyanate and chain extender used.
Soft segments have many chemical structures that are used in TPUs, and they give TPUs a huge range of properties because of the difference in the degree of interaction between SS and HS, and the chain flexibility of SS. The thermal and mechanical properties of TPUs are affected by variations in separation and domain size [18]. Micro-phase segregation in TPUs occurs between the hard-segment phase (HP) and the soft-segment phase (SP). This separation is incomplete, and the HP and SP still include certain amounts of the other segment [3][17]. As such, obtaining better mechanical properties of TPUs strongly depends on the phase at which segregation in segmented TPUs occurs, instead of the presence of hydrogen bonds [30].
Adjustments can made to soft and hard segments of TPU chains separately or simultaneously to achieve particular physical properties. These modifications can be carried out using different concentrations of hard segments and/or various kinds of polyols, chain extenders and isocyanates. For example, isocyanate reacts with an alcohol as a chain extender to produce urethane linkage, while urea linkage is produced by a reaction between isocyanate and an amine chain extender. As a result, the phase separation in diamine-extended poly(urethaneureas) is better than in diol-extended poly(urethanes) with the same diisocyanate [31]. Micro-phase segregation can be affected by the kind and conditions of the TPU-synthesis procedure, such as one-step or two-steps method and the reaction time between isocyanates and polyols as well as the various thermal histories that are applied to the TPU samples [3][17][18].
Miscibility between SS (low polarity and melting point) and HS (high polarity and melting point) is low, and the interaction between segments (SS and HS or HS and HS) plays an important part in determining the degree of micro-phase segregation [16][17][18]. The degree of phase segregation and domain formation rely on the content and nature of the soft and hard segments, on the molecular weight of the soft segments, on the kind of diisocyanate and polyol that are used to synthesise pre-polymers and on the sort of chain extender. SS represents flexible chains that promote elastomeric properties when they are segregated, while segregated hard segment domains, which are linked by hydrogen bonds, work as physical crosslinks inside the structure of TPU. Heat and solvents can dissociate hydrogen bonding of TPU that are returned by cooling or by evaporation of solvents [16][18]. As such, hydrogen bonds assist in promoting the total cohesion of the polymers [30].
There are four types of hydrogen bonds in polyether-based TPUs (see Figure 2.4) [32][33]. Hydrogen bonds form between hydrogen atoms and electronegative atoms such as nitrogen, oxygen, or fluorine. These four kinds appear due to the urethane linkages, which include three proton acceptors and one proton donator, while another proton acceptor is provided by the polyether soft segments [18]. H-bonds in the urethane linkage occur between N-H groups and (1) urethane alkoxy oxygen, (2) carbonyl group (C=O), (3) NH groups to form an NH… NH-bond, and (4) oxygen of an ether group (C-O-C). Interaction types 1, 2 and 3 occur between hard segments, while interaction type 4 occurs between hard and soft segments (see Figure 2.4) [32][33].
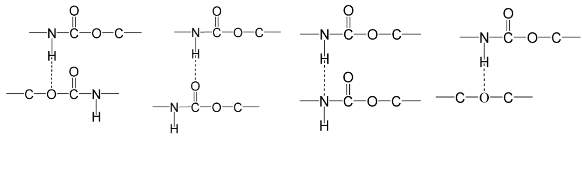 Figure 2.4: Four kinds of hydrogen bonds in polyurethanes based on polyether [32][33].
Figure 2.4: Four kinds of hydrogen bonds in polyurethanes based on polyether [32][33].
Fourier-transform infrared spectroscopy (FTIR) is a widely preferred technique to determine the hydrogen bonding in polyurethanes depending on observation of the absorbance of N-H and carbonyl stretching regions. The carbonyl or N-H stretching regions include hydrogen-bonded and free (non-hydrogen-bonded). The carbonyl stretching regions in polyurethane include a hydrogen-bonded carbonyl group at ~1702 cm-1 and a free carbonyl group at ~1721 cm-1 [18], while in polyurea these regions are a hydrogen-bonded carbonyl group at ~1630 cm-1 and a free carbonyl group at ~1690 cm-1 [34]. The N-H stretching regions in polyurethane are a hydrogen-bonded N-H group at ~3320 cm-1 and a free N-H group at ~3440 cm-1 [18], whilst in polyurea they consist of a hydrogen-bonded carbonyl group at ~3320-3340 cm-1 and a free N-H group at ~3450 cm-1 [34].
Hydrogen bonding in the polyurethane samples (pure HS) does not strongly reflect crystalline transitions (morphological transitions) inside the hard segment domains (during the melting of crystalline HS). Conversely, hydrogen bonding is affected by morphological transitions in the segmented TPU samples. These transitions are connected with inter-segmental mixing that results in the disruption of hydrogen bonds because of dissociation of HS domains [30][35][36]. As such, the glass-transition temperature of the hard-segment phase (TgHP) plays an important role in determining the thermal behaviour of the hydrogen bonds that dissociate above the TgHP.
Like most polymers, TPUs are highly sensitive to temperature. It is important to know how temperature changes can affect the properties of TPUs depending on their applications. TPUs have several thermal transitions that include glass-transition temperatures of the soft-segment phase (TgSP), hard-segment phase (TgHP) and mixed phase (TgMP), as well as the temperature of the micro-phase separation (TMST) and micro-phase mixing (TMMT). The exothermic peak of micro-phase segregation occurs because of segregation of the soft and hard segments in the mixed phase, in which they are completely separated. The endothermic peak of micro-phase mixing occurs if the hard and soft segments of TPUs are partially segregated [18].
Jeffrey T. Koberstein and Thomas P. Russell [37] used DSC and SAXS tests to study a set of samples of TPUs based on poly (oxypropylene glycol) end-capped with 30.4% (w/w) oxyethylene as a soft segment and a 4,4’- MDI chain extended with 1,4BD as hard segments. They suggested that the TPU samples had two temperature endotherms that included an intermediate-temperature endotherm (TII) and a high-temperature endotherm (TIII). TII related to the onset of micro-phase mixing of the soft-segment phase and the non-crystalline hard-segment phase [37].
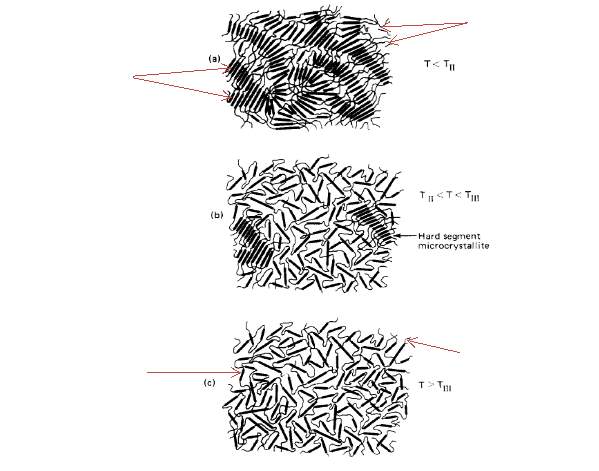
Figure 2.5:Morphological changes of TPU under heating: (a) T < TII; (b) TII < T < TIII; and (c) T > TIII [37].
This resulted from the transition of micro-phase segregation from an ordered to a disordered phase. Conversely, TIII related to melting of the microcrystalline HS. The morphological changes that occurred during DSC scans of the polyurethane were illustrated in a schematic model, shown in Figure 2.5 (a, b and c), where (a) applied temperature was below the micro-phase mixing temperature (T < TII); (b) applied temperature was between the micro-phase mixing temperature and the melting temperature (TII < T < TIII); and (c) applied temperature was above the melting temperature (T > TIII). The number of microcrystalline HS and ordered HS domains decreased with transition from (a) to (c). TII and TIII were affected by HS content and annealing temperatures[37].
The type and molecular weight of soft segments affect the extent of phase separation and the thermal and mechanical properties of the segmented TPUs [18][31]. Phase segregation of TPUs increases with increasing the molecular weight of soft segments [38]. Polyether or polyester polyols are mostly used in segmented polyurethanes. The hydrogen bonding between the ether oxygen of SS and N-H groups of HS is weaker than the interaction between ester carbonyl groups of SS and N-H groups of HS, resulting in less micro-phase segregation in TPUs based on polyester. It can therefore be said that TPUs based on polyether display greater resistance to hydrolysis and weaker temperature-related properties, while polyester-TPUs exhibit stronger physical properties [18].
The extent of phase separation and thermal and mechanical properties of segmented TPUs are also affected by the kind of isocyanate and the content of hard segments. For example, Tg of TPUs based on HDI and H12MDI increase with increasing the hard segment content, resulting in greater interaction among TPU chains (crystallisation of hard segments) [14]. TPUs based on HDI have a higher Tg than TPUs based on H12MDI due to the fact that HDI has a linear structure, unlike H12MDI, which has hexagon ring structures [14]. Crystallinity of TPUs increases with increasing the HS content [3][39][40][41][42][43]. The storage modulus of TPUs is improved [8][9][10][13][18][40][41][43][44] and TgHP shifts to a high temperature [9][10][13][40][42] with increasing the HS content. As the HS of TPUs increases, the yield strength, tensile strength and tensile modulus increase and the elongation at the break decreases [12][40][41][42][43][44][45][46]. This can be attributed to increasing the number of ordered HS with increasing the HS content, leading to an improvement in the number of H-bonds and interaction among TPU chains [39][40][41][47].
Sung et al. [31] carried out DSC, SAXS and WAXD tests on a series of samples of polyester poly(urethaneureas) and polyether poly(urethaneureas) based on a 2,4-toluene diisocyanate (2,4-TDI) chain extended with ethylenediamine (ED) as hard segments and poly(buty1ene adipate) (PBA) or poly(tetramethy1ene oxide) (PTMO) as soft segments. They investigated the effect of the urea linkage in the hard segments and the molecular weight of the soft segments on the extent of phase segregation and the thermal properties of the TPUs. The results showed that phase separation was improved with increasing the molecular weight of the soft segments from 1000 to 2000 g/mol polyesters or polyether. The extent of phase separation was higher in the polyether-based poly(ureathaneurea) (2,4-TDI-ED-PTMO) than in the polyester-based poly(ureathaneurea) (2,4-TDI-ED-PBA). Higher Tg in the soft-segment phase was seen in the polyester-based TPU than in the polyether-based TPU [31].
Seefried et al. [8] used a DMA test to investigate the effect of the molecular weight of soft segments on the dynamic mechanical properties of TPUs. TPU samples based on polycaprolactone diols (PCP) were used as soft segments with a molecular weight
( M̅n)range of 340 to 3130, and a 4,4′-diphenylmethane diisocyanate (4,4’MDI) chain extended with l,4-butanediol (1,4BD) as hard segments. The results displayed that the Tg of the TPU samples decreased with increasing the molecular weight of the soft segments. The authors suggested that soft segments with higher molecular weight were more mobile than soft segments with lower molecular weight.This was related to the superior phase separation of the TPUs when the higher molecular weight of soft segments was used [8]. The same authors [10] used the same technique to study the effect of the HS ratio and molecular weight of SS on the dynamic mechanical properties of TPUs. TPU samples based on PCP were used as soft segments with
M̅nof 830 and 2100, and a 4,4’MDI chain extended with 1,4BD was used for the hard segments. They reported that the structure and ordering of the HS strongly affected the dynamic mechanical properties of the TPUs. All TPU samples showed secondary relaxations related to the mobility of methylene alternation within SS. The Tg of the TPUs with 830
M̅nincreased by increasing the HS content, while the Tg of the TPUs with 2100
M̅nremained relatively constant for several different HS contents. The authors proposed that these differences were related to the degree of phase separation between the SS and HS in the TPU. TPUs based on low Mn SS displayed progressively high interaction between SS and HS domains at higher HS content compared with TPUs based on high Mn SS, which showed reduced interaction between the phases. The storage modulus of TPUs with 830 or 2100
M̅nincreased with increasing the HS content (see Figure 2.6) [10].
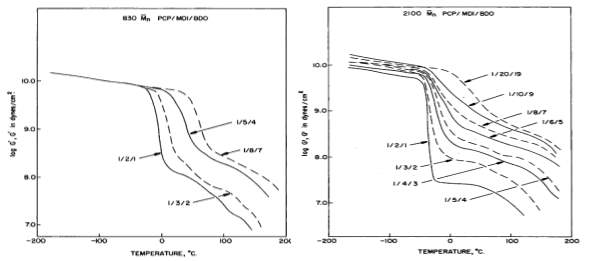
Figure 2.6: Effect of HS content on the storage modulus of TPUs: (A) with 830
M̅n; and (B) with 2100
M̅n[10].
B. S. Lee et al. [13] used a tensile test to study the effect of HS content on the tensile properties of TPUs. TPU samples were used based on poly(tetramethylene glycol) (PTMG) as soft segments and a 4,4’MDI chain extended with 1,4BD as hard segments with 20-50%wt HS. They reported that TPUs with high HS showed higher tensile strength and tensile modulus, and lower elongation at break (see Figure 2.7). They suggested that hydrogen bonds increased with increasing the HS content resulting in improving the interaction between TPU chains. TPU samples with high HS content proved difficult to stretch during the tensile test [13].
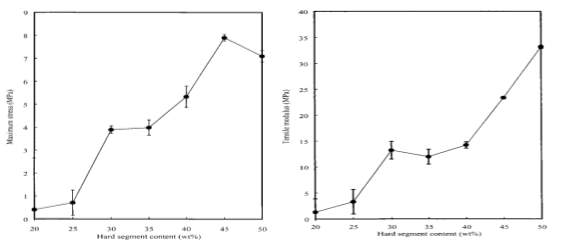
Figure 2.7: Effect of HS content on (A) maximum tensile stress and (B) tensile modulus of TPU [13].
The structure and length of chain extenders (CEs) also affects the extent of interaction between SS and HS [48][49]. The molecular weights of TPUs decrease with increasing the length of the chain extenders. This may be because the reaction of the pre-polymer with long CEs during polymerisation is more strained than with the short CEs. Therefore, short CEs yield TPUs with high molecular weights that have higher phase segregation and Crystallinity [48]. TPUs with long CEs have a lower Tg than TPUs with short CEs, indicating that a higher degree of phase segregation occurs in short CE TPUs [48][50]. In addition, the flexibility of TPUs increases with increasing the length of CE, resulting from increasing the mobility of the hard segments composed of diisocyanate and chain extender [50]. Camberlin et al. [51] used DSC to study the influence of the length of chain extender on the thermal properties of the TPUs. They used 1,2-ethanediol, 1,3-propanediol, 1,4-butanediol, 1,5-pentanediol, 1,6-hexanediol, 1,7-heptanediol, 1,8-octanediol and 1,10-decanediol as chain extenders. They reported that the TM of TPUs with 1,2-ethanediol, 1,3-propanediol and 1,4-butanediol were higher than for TPUs with other CEs [51].
The thermal history of segmented TPUs and the nature of soft and hard segments play an important role in determining the properties of TPUs. TPUs are affected by annealing treatment that promotes micro-phase segregation between soft and hard segments [3][18]. Crystallinity of TPUs increases [37][39][52][53][54] and TgHP shifts to a high temperature [18][55] with annealing treatment. The effect of annealing treatment on the structure of segmented TPUs was studied by Li et al. [56]. The TPUs used in this work were based on 4,4’MDI extended with 1,4 BD as hard segments with 50% HS content and poly(tetramethy1ene oxide) (PTMO) end-capped with poly(propy1ene oxide) (PPO) as soft segments. The annealing treatment enhanced phase segregation between hard segments within the soft segment matrix. They suggested that annealing treatment largely increased the interactions between hard segments. They concluded that mobility of hard segments and interaction between hard segments increased with increasing annealing temperatures, resulting in wide micro-phase separation [56].
Van Bogart et al. [52] used DSC to investigate the effect of the annealing treatment on the structure of segmented TPUs. The annealing temperatures used were -10, 20, 60, 90 and 120oC with annealing times of 4, 6, 12 and 16 days. They reported that annealing endothermic peaks were observed above the annealing temperature applied (between 20-50oC). Annealing treatment improved the degree of crystallinity of the HS segments. They explained that the annealing treatment encouraged disordered segments to reorganise themselves to become more ordered. The TPUs which had 100% HS showed the same results from annealing, so annealing endotherms did not rely on soft segments [52].
Jirakaittdual [18] studied the effects of annealing time (at 120oC) and various polyols: poly(tetrahydrofuran) (PTHF) and a poly(propylene oxide) end-capped with ethylene oxide (PPO-EO) on the properties of TPUs. A 4,4’MDI chain extended with 2-methyl-1,3-propanediol (MP-Diol) was used for hard segments with 65%wt HS. TPU-PPO displayed lower micro-phase separation than TPU-PTHF due to the increased interaction (lower immiscibility) between PPO soft segments and MDI/MP-Diol hard segments. It was concluded from the DSC and DMA results that higher micro-phase separation in both TPUs was produced by the longer duration (120 min) of the annealing treatment. The storage modulus (E’) and loss modulus (E’)’ of both TPUs increased with increasing annealing time (see Figure 2.8), while the intensity of the tan delta (δ) of TPU samples decreased with annealing treatment [18].
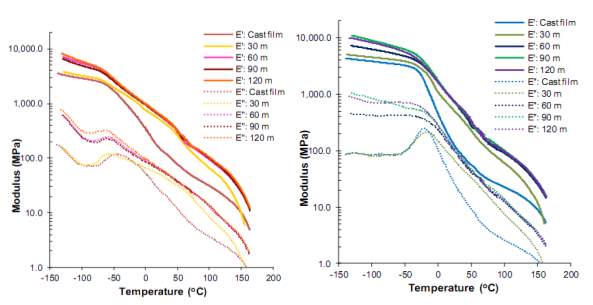
Figure 2.8: Effect of annealing time on the storage and loss modulus of (A) TPU-PTHF and (B) TPU-PPO [18].
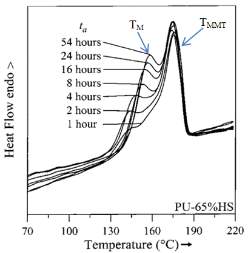
Saiani et al. [39] used DSC and WAXS to investigate a series of TPU samples with high HS content (50, 65, 75, 85, 90, 95 and 100%wt HS). Their TPU samples consisted of polyethylene glycol-block-polypropylene glycol-block-polyethylene glycol (EO-PPO-EO) as soft segments and a 4,4’-MDI chain extended with 2-methyl-1,3-propanediol (MP-Diol or 2M13PD) as hard segments. They studied the effects of annealing time (ta) at 120oCon the thermal behaviour of TPU samples. The results showed two endothermic peaks at high temperature in the TPU samples that were annealed several times. The first endotherm was at a lower temperature (melting transition of ordered hard segments present in the hard-segment phase) (TM), while the second was at a higher temperature (micro-phase mixing transition) (TMMT). The melting temperature (TM) and melting enthalpies (
∆HM) of TPU samples with 65%wt HS content annealed at 120oC increased with increasing annealing time (see Figure 2.9). The authors proposed that phase segregation and ordering of the hard domains increased with increasing annealing time [39].
Figure 2.9: Two endotherm peaks of TPU samples with 65%wt HS content annealed at 120oC for several different annealing times [39].
The same authors [47] reported that the melt-quenched TPU samples with 65%wt HS content had a two-phase structure, as seen in Figure 2.10 A. The first phase was a pure phase (hard-segment phase) and the second was a mixed phase (mixture of SS and HS) with 65% HS. The mixed phase might then undergo phase segregation, resulting in a phase-separated mesophase when the TPU samples were annealed at 120°C, as seen in Figure 2.10 B. TPU samples with HS content above 65%wt had a morphological structure consisting of soft-segment phase domains inside a continuous hard-segment phase matrix [47].
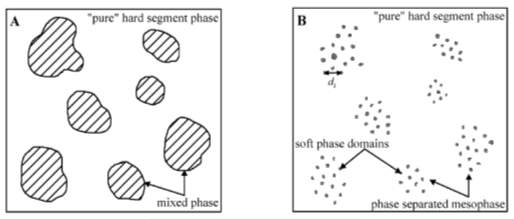
Figure 2.10: Morphological model of: (A) Melt-quenched TPU samples; and (B) micro-phase separated TPU samples [47].
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Chemistry"
Chemistry is a science involving the study of the elements and matter at the atomic and molecular level including their composition, structure, properties, behaviour, and how they react or combine.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please: