A Comparative Life Cycle Assessment of Precious Metal Catalysts: The Effect of Production Pathway
Info: 31532 words (126 pages) Dissertation
Published: 9th Dec 2019
Tagged: ChemistryOrganic Chemistry
A COMPARATIVE LIFE CYCLE ASSESSMENT OF PRECIOUS METAL CATALYSTS: THE EFFECT OF PRODUCTION PATHWAY
Contents
2.1 Green Chemistry & Green Engineering
2.2.1 Life Cycle Assessment Metrics
2.2.2 Challenges in Life Cycle Assessment
2.2.3 Life Cycle Assessment of Catalyst Nanosystems
2.3.1 Supercritical Fluids in Industry
2.3.2 Synthesis of Metal Oxide Catalysts from Supercritical CO2
2.4 The Water-Gas Shift Reaction
2.4.1 Current Lab-Scale Performance of Pt-CeOx/Al2O3 WGSR Catalysts
3.1 Life Cycle Assessment Phase Descriptions and Data Collection
3.1.1 Raw Material Extraction & Processing
3.1.4 Regeneration, Recycling, and Reuse
3.2 Life Cycle Assessment of Supercritical Fluid Deposition
3.2.1 Handling of Uncertainties & Sensitivity Analysis
3.2.2 Accelerated Life Cycle Testing
4.1 Raw Material Extraction, Processing, and Manufacturing
5.1 Product Development Phase Monte Carlo
5.3 Overall Product Life Cycle Monte Carlo
6.2 Description of Future Research
LIST OF TABLES
Table 1: Principles of green chemistry
Table 2: Principles of green engineering
Table 3: Comparison of WGSR catalysts
Table 4: User-defined materials and important assumptions for this study
Table 5: Nanosystem characterization data
Table 6: Highly accelerated life testing of nanosystems (table)
Table 7: Monte Carlo simulation results from product development phase
Table 8: Monte Carlo simulation results from product development phase with use phase contribution
LIST OF FIGURES
Figure 1: Contributions of design and development to environmental burden
Figure 2: Life cycle assessment system boundary
Figure 3: Relationship between design at the nanoscale and the mega-scale5
Figure 4: Does adherence to green design principles imply less environmental impact?22
Figure 5: Relationship between product life cycle emissions and environment burdens in ReCiPe 201641
Figure 6: Standard fuel processing system coupled with an SCF catalyst synthesis process
Figure 7: Lab-scale SCFD catalyst manufacturing vessel
Figure 8: System boundary for catalyst performance testing
Figure 9: Loss of exposed metal over time in a commercial automotive catalyst
Figure 10: System boundary of proposed study for competing catalyst preparation methods
Figure 11: Influence of feed composition, dispersion, and operating temperature on performance
Figure 12: Research organization
Figure 13: Comparison of Product Development Phases
Figure 14: Contribution of platinum related processes to human health (DALY)
Figure 15: Environmental burden with platinum content = 0, midpoint damage categories
Figure 16: Environmental burden with platinum content = 0, endpoint damage categories
Figure 17: Effect of platinum reduction in the SCFD product life cycle
Figure 18: Effect of energy recovery in SCFD product development (no platinum)
Figure 19: Recovery of energy in SCFD process w/ platinum
Figure 20: Impact of nanosystem performance on product development environmental burden (Midpoint)
Figure 21: Impact of nanosystem performance on product development environmental burden (Endpoint)
Figure 22: Highly accelerated life testing of nanosystems (graphical)
Figure 23: Influence of nanosystem robustness on production phase burden (midpoint)
Figure 24: Influence of nanosystem robustness on production phase burden (endpoint)
Figure 25: Effect of platinum recovery on product development
Figure 26: Monte Carlo simulation results for product development phase (graphical)
Figure 28: Monte Carlo simulation of overall product life cycles with performance contribution
ABSTRACT
The objective of this research is to illustrate the need to consider the environmental burden and impact posed by the adoption of alternative, green process technologies. Specifically, it addresses the synthesis of catalysts using an alternative, environmentally benign solvent. Using the life cycle assessment methodology, a quantitative technique that assigns damage metrics to process emissions, the potential environmental damage is estimated. An extensive literature search validates the uniqueness and highlights the scientific merit of the research. Data gathered includes background information on supercritical processes, life cycle assessments of systems, aggregated in-house catalyst performance data, and a case study for catalyst application.
Catalyst synthesis via supercritical fluid deposition possesses several advantages over traditional techniques that can reduce production costs and environmental burden while maintaining high activity. The creation of catalysts using a “green” approach reduces harmful emissions but also drives the advancement of alternative fuel technology, an area known to make use of expensive catalytic materials. Additionally, this research provides a rationale for adoption of supercritical CO2 at the commercial level for metal-oxide supported catalysts. This study demonstrates how improvements at the nanoscale propagate upwards through a product development system, sometimes with unpredictable outcomes. Furthermore, it illustrates the benefit of a holistic approach to chemical process design, an area that strives for a balance between economics, performance, and environmental sustainability.
CHAPTER I
INTRODUCTION
Within the engineering profession, there is an inherent moral obligation to preserve resources and implement policies and processes that benefit future generations and humanity. Presently, research in chemical engineering stresses the use of environmentally sustainable processes through “green chemistry” and “green engineering”.1,2 Supercritical fluid deposition (SCFD) from CO2, a catalyst preparation technique, is frequently touted as a green process, typically because of the relatively mild reaction conditions and the use of an environmentally benign solvent. These aspects should inherently leave a small carbon footprint. Despite these claims, there is a lack of evidence, qualitative or quantitative, evaluating what the environmental impact truly is. The figure on the following page highlights why it is advised to understand the process design fully before implementation.3
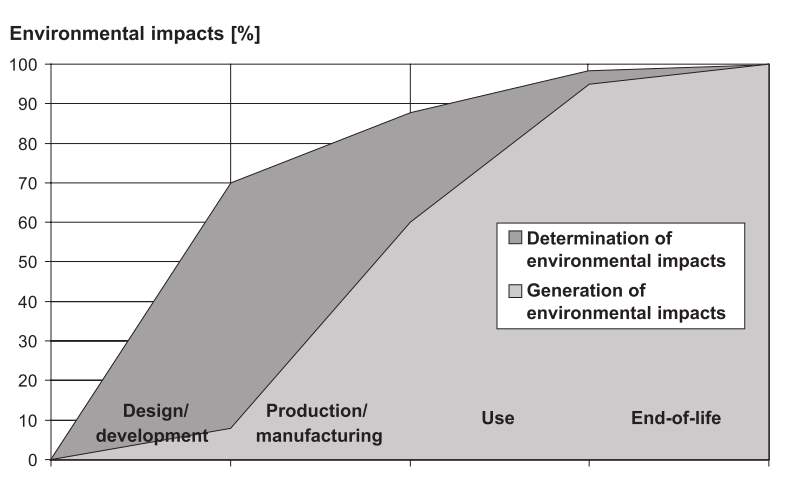
Figure 1: Contributions of design and development to environmental burden
Less than 10% of the overall environmental impact occurs during design and development, yet 70% of the impact is quantified and sourced. Time is allocated at the beginning of a product or process design to reduce and/or mitigate future outputs.
The aspects of the SCFD process mentioned above are not the only metrics that define a “green” process and are specifically focused on one stage of the product life cycle. Thus, they fail to take into account the quality of the catalyst/product being produced along with possible reuse and/or other end of life aspects. To build a case for this method’s viability as an alternative synthesis route, its overall environmental impact through a life cycle assessment (LCA) must be performed. It is important not to confuse the system life cycle with an environmental life cycle assessment (usually referred to simply as an LCA). Typical system life cycle stages along with their purpose and possible gate decisions at each stage are given in Table 1.
Table 1: System life cycle stages
| Life Cycle Stages | Purpose | Decision Gates |
| Concept | Identify Stakeholder Needs | |
| Explore Concepts | ||
| Propose feasible solutions | Decision Options: | |
| Development | Refine system requirements | > Execute next stage |
| Create solution description | > Continue this stage | |
| Build system | > Go to previous stage | |
| Verify and validate system | > Hold project activity | |
| Production | Mass produce system | > Terminate project |
| Inspect and test | ||
| Utilization | Operate system to satisfy users’ needs | |
| Support | Provide sustained system capability | |
| Retirement | Store, archive, or dispose the system |
Table 1 is a simplified and truncated version of the system life cycle available in the NASA Systems Engineering Handbook.4 An environmental life cycle assessment is an element of the “Concept” stage, useful in alternatives analysis and trade studies. However, data generated from each system life cycle stage and lessons learned are gathered to enhance future life cycle assessments. For this study, only the environmental life cycle assessment is of interest although ramifications from the results will impact system life cycle decisions.
The development of life cycle assessment methods arose during the 1970s with the 1990s seeing a concerted effort by numerous organizations to develop a standardized LCA methodology.5 The definition of a life cycle assessment is given by Rebitzer et al:3
“Life cycle assessment (LCA) is a methodological framework for estimating and assessing the environmental impacts attributable to the life cycle of a product, such as climate change, stratospheric ozone depletion, tropospheric ozone (smog) creation, eutrophication, acidification, toxicological stress on human health and ecosystems, the depletion of resources, water use, land use, and noise—and others.”
The framework for the life cycle assessment and the system boundary is shown in Figure 2.
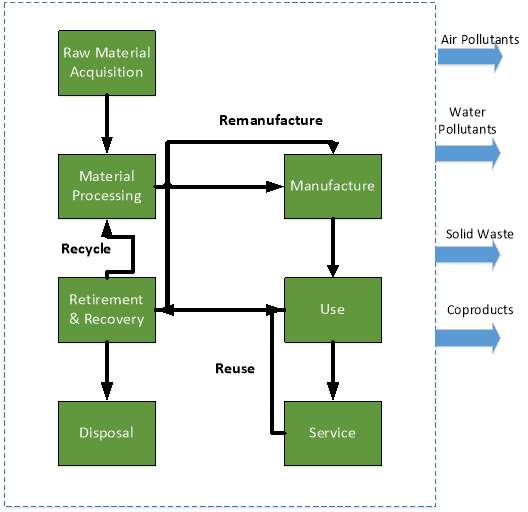
Figure 2: Life cycle assessment system boundary
Figure 2 is a segmented and a compartmentalized view of a systems/products life cycle. As mentioned earlier, there is significant overlap between the stages in a life cycle assessment and a system life cycle. A key difference is that an LCA investigates physical consequences that may occur from decisions made during the conceptual stage. This relates back to Figure 1, where the majority of environmental burden can be quantified and source during early system life cycle stages.
To produce any product, energy is the primary concern. This energy includes all processes required to fabricate the product, ranging from mining operations to recycling and/or reuse. From these processes, the outputs will always include unwanted products such as pollutants, emissions, and other wastes. These outputs can be linked through a series of assumptions and calculations to quantified potential damages, those being disability adjusted life years (DALY), potentially disappeared species x year, and depletion of mineral resources (USD2013). Each of these measurements are commonly accepted indicators for damage to human health, damage to ecosystem quality, and damage to mineral and fossil resource availability.6 Of these categories, does supercritical fluid deposition from CO2 reduce these environmental burdens?
Thermal deactivation or agglomeration of platinum particles over a catalyst’s life is a major contributor to performance loss.7 As such, the ability of supercritical CO2 to deposit highly dispersed, strongly anchored nanoparticles is significant. The link between processes at the nanoscale and that at the macro-scale and mega-scale is illustrated in the figure on the following page from Charpentier.8
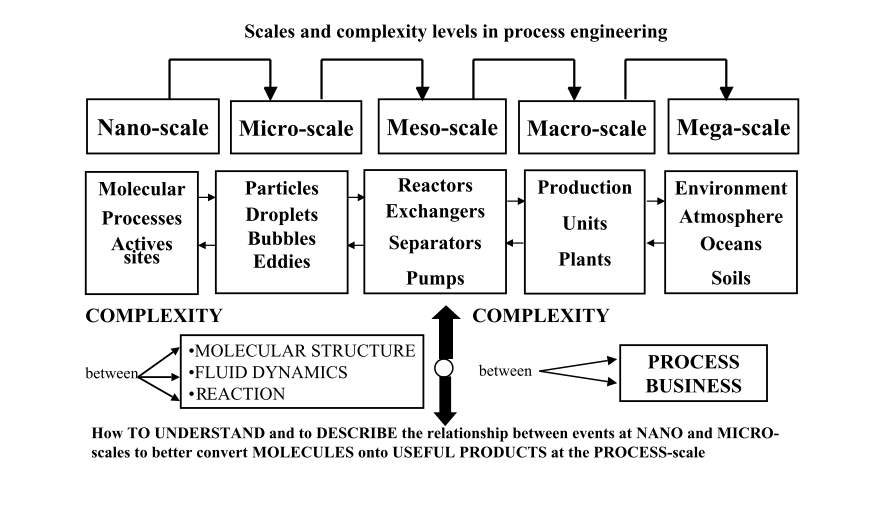
Figure 3: Relationship between design at the nanoscale and the mega-scale8
Figure 2 shows the relationship between nanoscale properties and how their manipulation ultimately affects global issues. A life cycle assessment can provide an environmental-oriented rationale for the adoption of supercritical fluid deposition at an industrial level for the synthesis of supported metal-oxide nanosystems.
1.1 Research Statement
There are several studies that identify supercritical fluid deposition using carbon dioxide as a “green” method for chemical processes, including catalyst synthesis.9–12 However, there is no quantification of how “green” the process is. Much of the carbon dioxide produced currently is an inevitable byproduct from the combustion of fossil fuels and other carbon containing compounds. Realizing this, the material balance of any process that uses CO2 as the solvent will inherently have near zero net carbon output and have little contribution to the total CO2 already in existence. However, the complexity of a systems life cycle necessitates a larger view. To understand the complete picture of the potential environmental impact posed by a supercritical process, a life cycle assessment is necessary.
1.2 Research Hypothesis
Hypothesis: Alumina-supported platinum/ceria catalysts for water-gas shift reaction prepared from a supercritical fluid deposition system contribute less potential environmental damage than those prepared by incipient wetness impregnation
Three damage metrics define potential environmental damage for this study:
- Disability adjusted life years (DALY)
- Potentially disappeared species x year
- U.S.A. Dollars (2013)
These units are common for life cycle assessment studies.
1.3 Research Objectives
Several research objectives frame this study:
- Quantify the environmental emissions of a supported metal oxide catalyst prepared by supercritical fluid deposition
- Quantify the environmental emissions of a supported metal oxide catalyst prepared by incipient wetness impregnation
- Determine the potential environmental damage of these catalysts over their entire product life cycle using three metrics
- Disability adjusted life years (DALY)
- Potentially disappeared species x year
- U.S.A. Dollars (2013)
Summarized, the objective of this research is to evaluate the potential environmental impact and burden that a precious metal-based catalyst prepared from supercritical fluid deposition presents to the environment. Regardless of which solvent and synthesis path employed, it is impossible to have zero impact on the environment. However, by making a quantitative assessment of SCFD’s impact as an alternative route for catalyst synthesis, it is possible to reduce harmful environmental outputs while maintaining high catalytic activity. It is also possible to apply the results of this work to other metal oxide nanosystems prepared using supercritical fluid deposition. This study serves as a starting point and/or source of data for other similar LCA studies.
1.4 Research Contributions
There is a scarce amount of published work involving supercritical processes and their environmental impact. An LCA study with a gate-to-gate scope was performed in 2012 that compares alternative methods of antioxidant extraction from rosemary to supercritical fluid extraction.13 The supercritical solvent in this study was a combination of CO2 and ethanol. Another LCA study, performed in 2016, cradle-to-gate only, uses supercritical CO2 to prepare barium strontium titanate nanoparticles. Both of these studies draw similar conclusions, that the major contributor of environmental burden from a supercritical process is the energy requirements. This research is the first to include the performance of a product prepared from supercritical fluid deposition in a life cycle assessment.
In a broader sense, a life cycle assessment pinpoints areas where refinement and improvements can reduce the environmental impact of catalyst synthesis processes. The comparisons of SCFD to traditional catalyst synthesis methods provides an understanding of environmental trade-offs involved with transitioning to a novel synthesis approach. This research lends support towards SCFD adoption on a larger, industrial scale.
This research contributes to the growing field of systems engineering at the nanoscale, and provides an example of the interdisciplinary nature of systems engineering. Studying nanoscale interactions can benefit top-level design while minimizing global environmental impacts. Through an in-depth study of a particular catalyst nanosystem, the conclusions and lessons learned can be applied to other supported metal oxide nanosystems prepared from supercritical CO2. There has not been a life cycle assessment, a cradle-to-grave study, of supercritical fluid deposition as an alternative synthesis process for platinum group metal-based catalysts. Cradle-to-grave analyses embody systems engineering, with frameworks existing that can be applied to manage any system.14 This research tackles not only work at the nanoscale, but analyzes how optimization of material use and “green” technology benefits local, regional, and global environmental concerns.
CHAPTER II
LITERATURE REVIEW
Chemical engineering has become much more complex with “safety, health, environmental aspects, including non-polluting technologies, reduction of raw material and energy losses and product/by-product recyclability” all being factors in system development.15 As stated by Charpentier et al., “simple economics are no longer the defining factor in the production of a product”.8 This increase in complexity requires a systems thinking or holistic approach. Questions such as “How does implementation of a ‘green’ synthesis pathway affect product and overall system properties?” and maybe more importantly, “Are these changes necessary and/or beneficial?” are necessary for process and product development.
The Intergovernmental Panel on Climate Change reports that it is “extremely likely that more than half of the observed increase in global average surface temperature from 1951 to 2010 was caused by the anthropogenic increase in GHG concentrations and other anthropogenic forcings together”16. Therefore, even more so for those in the engineering profession, reducing the carbon footprint and harmful environmental emissions from chemical processes is an important and morally justifiable goal. Furthermore, current emission and pollution policies, along with increased public awareness of environmental ramifications, dictate environmentally conscientious products and processes. However, before implementation of a “green” or novel process, there must be a quantifiable and demonstrable rationale.
The goal of this literature review is to provide background information on a number of areas of this research, from a broad system-level viewpoint, down to molecular level, nanosystem behaviors. The rise of green chemistry in chemical engineering and catalysis is reviewed, along with processes typically used today for the production of metal-oxide catalysts. The history and implementation of life cycle assessment to systems and its relevance to chemical engineering is investigated. For the application of the catalyst of interest, a case study involving hydrogen generation for use in a typical fuel cell vehicle is studied. Specifically, the manufacturing and use phases of the catalyst are reviewed, as a priori knowledge predicts they are the most affected by a change in the manufacturing process. However, because of the summative nature of the LCA, all areas of the system life cycle are addressed.
2.1 Green Chemistry & Green Engineering
Green chemistry is defined as:1
“The use of chemistry techniques and methodologies that reduce or eliminate the use or generation of feedstocks, products, by-products, solvents, reagents, etc., that are hazardous to human health or the environment.”
There are twelve principles of green chemistry or “design rules” for chemists proposed by Anastas in 1998,17 with some referring to them as a “reaction toolbox”.18
Table 2: Principles of green chemistry
| Prevention | Use of Renewable Feedstocks |
| Atom Economy | Reduce Derivatives |
| Less Hazardous Chemical Synthesis | Catalysis |
| Designing Safer Chemicals | Design for Degradation |
| Safer Solvents and Auxiliaries | Real-Time Analysis for Pollution Prevention |
| Design for Energy Efficiency | Inherently Safer Chemistry for Accident Prevention |
These principles are viewed as a framework/guideline for chemical engineers. They look to maximize energy and material efficiency while simultaneously minimizing material waste, mitigating process risks, reducing harmful environmental impact, and using sustainable resources. These principles were eventually generalized for all engineering disciplines, producing the twelve principles of green engineering.2
Table 3: Principles of green engineering
| 1. Designers need to strive to ensure that all material and energy inputs and outputs are as inherently nonhazardous as possible. |
| 2. It is better to prevent waste than to treat or clean up waste after it is formed. |
| 3. Separation and purification operations should be designed to minimize energy consumption and materials use. |
| 4. Products, processes, and systems should be designed to maximize mass, energy, space, and time efficiency. |
| 5. Products, processes, and systems should be “output pulled” rather than “input pushed” through the use of energy and materials. |
| 6. Embedded entropy and complexity must be viewed as an investment when making design choices on recycle, reuse, or beneficial disposition. |
| 7. Targeted durability, not immortality, should be a design goal. |
| 8. Design for unnecessary capacity or capability (e.g., “one size fits all”) solutions should be considered a design flaw. |
| 9. Material diversity in multicomponent products should be minimized to promote disassembly and value retention. |
| 10. Design of products, processes, and systems must include integration and interconnectivity with available energy and materials flows. |
| 11. Products, processes, and systems should be designed for performance in a commercial “afterlife”. |
| 12. Material and energy inputs should be renewable rather than depleting. |
Utilizing these guidelines, the application of a life cycle assessment studies their effect on a process, product’s or system’s environmental impact.
2.2 Life Cycle Assessment
Traditionally, economic criteria have been used as the basis for process decisions.19 However, rising interest in sustainable energy sources has led to increased investment in “green” technology.20 The application of a life cycle assessment to these systems is beneficial in identifying key areas for optimization and in investigating the potential of alternative technologies. It is most useful in situations where both upstream and downstream inputs/outputs are known and all areas of the system life cycle are quantified. Life cycle assessment (LCA) is a methodology that assesses the potential environmental impact of a process or product from cradle to grave. The purpose of a life cycle assessment is to aid in achieving three specific goals. Hofstetter lists them as:6
- To generate as many services as possible with the least amount of products
- To produce these products with a minimal material flow
- To reduce as much as possible environmental interventions per industrial throughput
An LCA can identify areas for refinement with regard to emissions or pollutants and highlight areas for improved energy and material use.
There are a number of LCA studies that focus on process and products.21,22 Some compare the environmental impact of alternative technologies to traditional, such as cement manufacturing that employs carbon sequestration,23 while others address the environmental feasibility of existing technologies, such as hydrogen production from electrolysis.24 An example in line with “green” energy is a review by Peng et al. of solar photovoltaic (PV) systems and their environmental emissions.25 Solar PV systems are examples of a technology that conjures the idea of being inherently and completely environmentally friendly, specifically in the “use” phase, but expansion of the system boundary demonstrates this to be somewhat misleading when accounting for material extraction, manufacturing, disposal, etc. The principles of green chemistry do not always reconcile with a life cycle assessment. Figure 4 highlights this conundrum and why the entire life cycle of the product needs consideration.
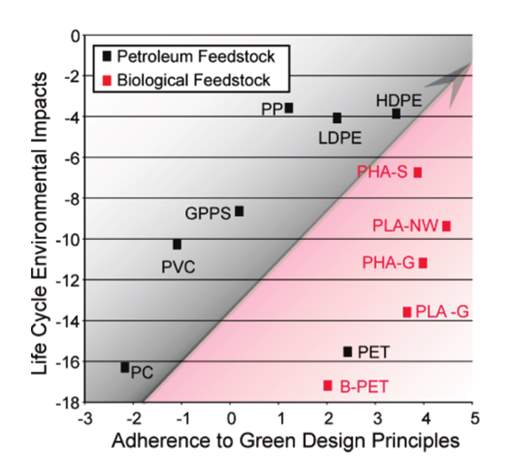
Figure 4: Does adherence to green design principles imply less environmental impact?26
Figure 4, from a study by Tabone et al., shows the relationship between environmental burden and adherence to green design principles for petroleum/biological feedstocks for plastic production.26 Although a product may adhere to the principles, it is not necessarily the best environmental choice when looked at from a larger perspective. The complex relationships between systems and the environment has shown multi-objective trade-off analysis to be necessary when performing life cycle assessments, especially when attempting to maximize the performance of a product while minimizing the environmental impact or the economic costs.19
Measuring a system’s environmental burden can be daunting. This sometimes makes execution of life cycle assessments a challenging and uneasy task. This is partly due to the sheer magnitude of variables involved in a product’s life cycle, the needed assumptions, and/or recognizing the environmental primary, secondary, and tertiary effects. For these reasons, it is common to focus LCA studies on specific phases of the life cycle, depending on the goals of the analysis.27
2.2.1 Life Cycle Assessment Metrics
Life cycle assessment has met criticism during its development due to the amount of subjectivity in its methods, particularly in the impact assessment, which establishes a criterion for how to quantify emissions and abstract environmental concerns to environmental damages. Cumulative energy demand and associated greenhouse gas (GHG) emissions (typically coupled),28–30 energy payback time,31,32 and environmental, health, and safety (EHS)33 are all possible metrics utilized in studies, many times in combination to paint a clear and accurate picture. Rather than reducing the environmental impact to a single measure, which can mask or hide certain environmental concerns, the effort has been made to add multidimensionality by the introduction of impact categories. Examples include climate change, acidification, eco-toxicity, eutrophication, etc. These environmental concerns, lacking a numerical value, are instead classified and seen as “midpoint” indicators or abstract concepts that produce a consequence. Because of their inherent qualitative and conceptual nature, the introduction of “endpoints” is necessary. These “endpoints” are damage categories, the potential ultimate outcome of the accumulation of environmental emissions and disturbances.
The life cycle impact assessment (LCIA) captures a quantitative understanding of the life cycle inventory phase of an LCA, providing the assumptions and calculations necessary to link “midpoints” and “endpoints”. Software provides practitioners of LCA the tools necessary to conduct independent studies of their processes and/or products, identifying environmental “hotspots” and reducing inefficiencies. SimaPro, developed by PRé Sustainability, is one of the leading LCA software packages, used in both industry and academia.22,23,27,28,34–39 SimaPro makes use of life cycle inventory databases to complete life cycle assessments, and relies on several user-specified weighting methods to quantify results. The ecoinvent database is the current leading life cycle inventory database and is bundled and sold with major LCA software suites. Its transparency and comprehensive data make it a popular choice used in industry, with large companies such as Procter & Gamble and Nestlé implementing it in their own internal life cycle assessments.40,41
There are numerous methods that analyze the life cycle inventory and establish links to environmental burdens and quantified damages, with some taking a midpoint approach and others looking into endpoint categories.37,42,43 A well-developed method known as ReCiPe, attempts to harmonize the relationship between these two valid approaches.44 Based on numerous assumptions and calculations, ReCiPe takes abstract environmental concerns, such as climate change and ozone depletion, and relates them to three quantified damage categories. These groupings include damage to mineral and fossil resources, damage to ecosystem quality, and damage to human health. The figure on the following page shows the relationships that ReCiPe establishes between the outputs of a product’s life cycle phases and the potential environmental burden.44
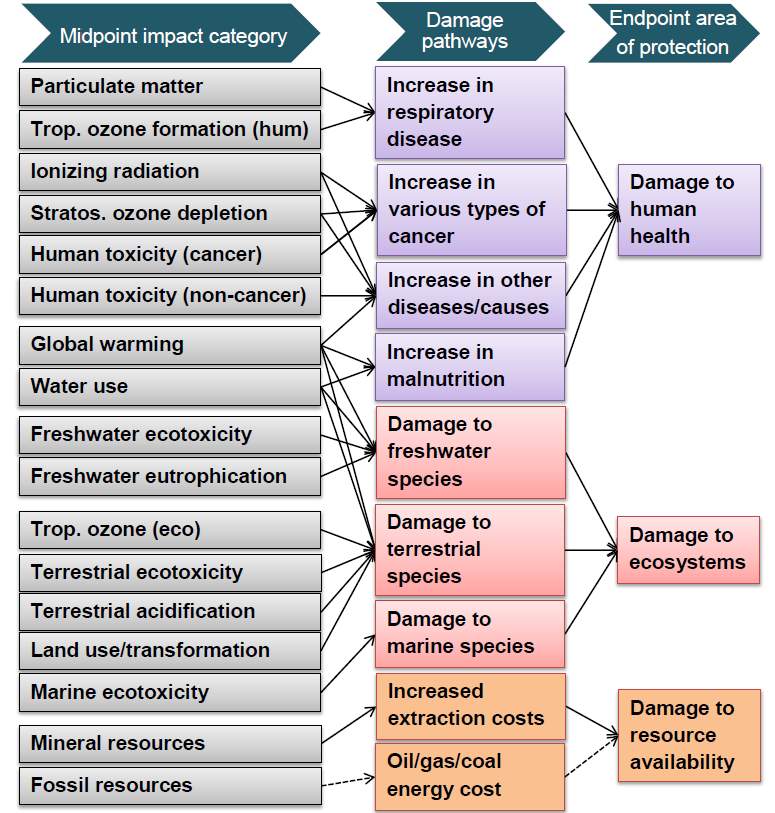
Figure 5: Relationship between product life cycle emissions and environment burdens in ReCiPe 201645
Figure 5 draws the relationships between emissions, land use, and resource use. In this impact assessment method, there are three final, numerical scores given: damage to human health (disability adjusted life years), damage to mineral and fossil resources (USD2013) and damage to ecosystem quality (potentially disappeared fraction of species x year). It is important to note the role of subjectivity in this impact assessment. The sheer number of assumptions introduces a relatively large degree of uncertainty, increasing more so as the 17 midpoint categories are merged into three endpoints. The methodology makes use of cultural theory to address this, using three view different perspectives with different assumptions: individualist, hierarchist, and egalitarian. Each perspective differs in time horizon considerations and toxicity assumptions. Further information regarding this impact method and how it draws these relationships is available in the ReCiPe methodology report and the Eco-indicator 99 methodology report.44–46
2.2.2 Challenges in Life Cycle Assessment
Life cycle assessment is a useful and powerful tool; however, it is not without its faults. There are two major areas for improvement: data uncertainty and modeling uncertainty. A critique by Ayres from 1995 cites a shortfall in life cycle assessment being the lack of verifiable, transparent, and published data.47 Furthermore, it addresses the absence of a mass balance in life cycle assessment. These concerns still exist; however, work has been undertaken to mitigate, mainly in the expansive and transparent ecoinvent database which allows tracing of material and energy flows.41 A more recent critique (2008) by Reap et al. cites similar problems in life cycle assessment, the major concerns being the definition of environmental boundaries (truncation), environment uniqueness (non-homogeneity), and the subjective nature of choosing midpoint and endpoint indicators.48 There can be disagreement between different software conclusions as well, demonstrated in a study by Herrman et al.34 The study found variance between two leading LCA software packages, and attributed the difference to how the software handled impact assessment methodologies. There is ongoing work to reconcile and harmonize LCA studies, with attempts so far including ISO standards to standardize the methodology.49 Ultimately, the researcher, consumer, or stakeholder dictates what metric is most appropriate and important, while understanding the limits and assumptions of the LCA. It is essential for practitioners to clearly state the assumptions made for their analysis. Life cycle assessment is a decision-supporting tool, not the ultimate dictator for what is the correct choice.
2.2.3 Life Cycle Assessment of Catalyst Nanosystems
Nanosystems share the same definition of a system but differ from micro and macro scale systems due to the unique properties they exhibit and the complex interactions that occur at their interfaces. As technologies have advanced, manipulation of the atomic structure of a nanosystem has become possible, allowing adjustment and tailoring of properties to accomplish a specific task. From an environmental perspective, this has resulted in less material use and subsequently, less waste and emissions. This level of precision and control has led to the use of nanosystems in several areas including drug delivery systems,50,51 solar cells,52 separation processes,53 and advanced materials.54 Additionally, this progress has led to an increased understanding of molecular interactions at their interfaces, leading some to designate multicomponent catalysts as nanosystems.15,54–58 With this progress, nanoscale work has been stressed in catalyst synthesis;59 however, relatively few works address catalyst and/or nanotechnology from an environmental perspective.
It is essential to recognize that nanoscale improvements will ultimately affect a system as a whole. With an increasingly environmentally aware public and the need for safe processes, consumers and the market have new product requirements. Consequently, the need exists to take a multidisciplinary and multiscale approach to these changes, with life cycle assessment an established method to manage these complex interactions.8,15 Some researchers have taken note of this, applying life cycle assessment to catalytic research at the nanoscale. Platinum group metal (PGM) reduction has been investigated in automotive catalysts using life cycle assessment.60 The study is more of a “what if” scenario, drawing conclusions from the hypothetical continued improvement of nanotechnology, but provides ample reasoning for why PGM reduction is beneficial, important, and a worthwhile investment. However, this study is constrained to raw material extraction. Most other LCA studies involving catalysis follow this format, using life cycle assessment to gauge what performance metrics and properties future catalysts should exhibit.61 A study by Holman et al. looks at using life cycle assessment to guide catalysis research.61 The study compares a cheaper but less efficient feedstock, propane, to the current and high-yield process for producing propylene. The conclusions are interesting in that they do not necessarily support implementing propane, but provide a theoretical “break even” point for engineers to strive towards. Dealing with costs and environmental impact is an intricate and complex balancing act. By achieving a yield of 60% through catalyst improvement, compared to a yield of 87% for propylene, propane becomes economically viable while also having a lower environmental impact. It should be noted that at lower yields (6%), propane is not economically viable but is environmentally favorable, an example of how the implementation of “greener” processes can be stifled by economic constraints.
Life cycle assessment is also used to address material selection for composites and in solvent selection when multiple criteria are to be satisfied.33,62 The environmental impacts of nanosilver t-shirts have been investigated, with the study going so far as to consider the potential health effects of the nanomaterials themselves.63 Carbon nanotubes prepared from chemical vapor deposition have also been studied to identify environmental “hot spots”.22 The study addresses the lack of LCA studies on nanotechnology. The study cites that only 7 out of 16,500 hits for “nanotechnology” contained any mention of life cycle assessment. Furthermore, of these studies, much uncertainty was present due to lack of data concerning nanomaterials. This agrees with the findings that most LCA studies involving catalysis and new technologies are anticipatory only, with some addressing only certain phases of the life cycle due to life cycle inventory uncertainties.
When performing assessments involving nanotechnology, data are unavailable at times, either due to the novelty of the technology or because of industry patents. Although the exploitation of nanotechnology can reduce material use significantly,60 the environmental and human toxicological effects remain difficult to discern, leading to a higher degree of uncertainty in the impact assessment.63–65 It is crucial to identify assumptions made to correctly interpret results. Furthermore, when comparing nanomaterials with traditionally produced products, some recommend stressing the toxicological aspects of the nanomaterial over other categories, with a much higher performance of the nanomaterial required for a “go” decision.66 Much of the data for this research was available within European and North American databases utilized by SimaPro, with the remainder collected in-house and from literature..
Literature shows that a life cycle assessment of the production of metal oxide catalysts, specifically the Pt-CeOx/Al2O3 nanosystem via supercritical fluid deposition, has not been performed. In fact, complete life cycle assessments of supercritical fluid processes in general are lacking; surprisingly, there is an LCA study that has been conducted by Tsang et al. involving the synthesis of barium strontium titanate nanoparticles from supercritical CO2.39 The study is limited to a cradle to gate window, not taking into account the use phase or any end of life considerations. Supercritical fluid deposition is a viable alternative production method because of its ability to produce a high quality product, a recurring characteristic of processes that make use of supercritical carbon dioxide.
2.3 Supercritical Fluids
Supercritical fluids (SCF) are fluids subjected to pressures and temperatures beyond their critical parameters. In the near supercritical region, the SCF displays properties characteristic of both a liquid and a gas.67 They possess densities in the range of liquids yet retain the diffusivity of gases. The ability to tune properties of the SCF towards more liquid-like properties or gas-like properties by slight changes in either the pressure or temperature extends their practicality. These unique properties make supercritical fluids an attractive means for catalyst synthesis.
Carbon dioxide is the leading solvent for SCFD because it is environmentally benign, non-toxic, nonflammable, and has a relatively accessible critical point. Rates of deposition are higher due to an increase in mass transfer, liquid waste is minimal, and the substrate contains no solvent residue.68 Characteristics of supercritical fluids that facilitate deposition processes include the ability to dissolve a wide range of organometallic precursors, high mass transfer rates, zero surface tension, and miscibility with reducing gases.69
2.3.1 Supercritical Fluids in Industry
Currently, the main industrial use of supercritical fluids, specifically supercritical CO2, is within the food industry and extraction processes in particular.70–72 An example is the relatable and well-known decaffeination of coffee beans and tea.73,74 The drive towards supercritical CO2 as the solvent of choice for these processes has been mainly from public pressure and environmental regulations and the desire to use more “natural” solvents.75 Of the few economic analyses available, the main cost of implementation of these processes is the initial capital investment; however, this is mitigated by high product recovery and reduced wastes.76 Furthermore, for many green processes, it has been shown that the main contributor of environmental burden is the primary energy source for the process,13 although this can fluctuate dramatically if the energy source is a mix and/or renewable.77 These examples demonstrate the intimate connection between energy consumption, process efficiency, and waste disposal along with the added complication of temporal/spatial dimensions. It is vital to approach the development of new technology and chemical processes from a holistic, or rather a “systems thinking” mindset.
2.3.2 Synthesis of Metal Oxide Catalysts from Supercritical CO2
Metal oxide catalyst preparation traditionally involves exposing support material to a solution containing the dissolved metals where the dissolved metals then adsorb to the surface of the oxide.78–86 The need for a liquid solvent and post-synthesis processing leads to an increase in waste generation and energy use. Furthermore, the deposition of metals can be highly inefficient resulting in increased material use, lower performance, and possible overburdening during the disposal phase. The disadvantages of these methods are established.87 Supercritical fluid deposition offers advantages and benefits that make it a viable alternative, both from environmental and performance perspectives for catalyst synthesis.
Synthesis of metal oxide catalysts using supercritical fluids has innate advantages over conventional methods. Preparation techniques used today are based on wet impregnation which leaves behind impure solvent and a product that requires additional chemical treatment.88 Furthermore, this method shows poor control of noble metal particle size leading to inefficient material use and non-optimized performance. Comparing to SCFD with CO2 as a solvent, liquid waste is non-existent and after deposition, there is no leftover solvent residue on the product itself. This eliminates the need for additional purification processes that may negatively influence the product. Lack of surface tension results in a more even distribution of noble metals on the surface of the metal oxide, resulting in efficient material use.89 Solvation of the noble metal precursors is also important, with a number of organometallics possessing low solubility in scCO2.90 Supercritical CO2’s miscibility with reducing gases alleviates additional equipment use as well.91
The typical solvent of choice for deposition on metal oxides is supercritical carbon dioxide. It is the leading solvents for supercritical fluid deposition because it is environmentally benign, relatively inert, non-toxic, nonflammable, and widely available. The accessibility of the critical point of CO2 is important as well, especially in the context of energy requirements. The use of CO2 as a solvent will greatly influence the manufacturing/synthesis phase of the life cycle. The characteristics of catalysts produced from supercritical CO2 are without question one of its strongest attributes, ultimately playing a role in the use phase of the catalyst.92
Particle size, metal distribution and the concentration of the metal being deposited are key parameters that influence the activity of the catalyst.93 SCFD of platinum on an alumina support using supercritical CO2 has been shown to result in consistent and even deposition of platinum nanoparticles with average particle sizes ranging from 1.2 – 2.7 nm.93 Deposition occurred over the course of 24 hours at 27.6 MPa and 80°C in a sealed, stainless steel vessel. The authors note how the concentration of the metal affects the particle size and distribution with higher concentrations leading to larger particle sizes from increased occurrences of coalescence. The interactions between the support and the metallic precursor also appeared to influence the size of the particles and their distribution. Use of silica supports resulted in larger platinum nanoparticles and an inconsistent metal distribution compared with alumina supports. This difference was attributed to the strength of the interactions between the organometallic precursors and the support. SCFD of platinum on alumina foam-supported SnO2 was performed to generate a catalyst for the preferential oxidation (PROX) of carbon monoxide, another important reaction for CO minimization in a hydrogen production system.94 Compared to a similar catalyst produced by wet deposition, the SCFD proved to be the superior method as the maximum particle diameter exhibited was no larger than 7 nm, while particles on the catalyst prepared by wet impregnation had an average diameter of 27 nm. Furthermore, because the reaction takes place at the interface between platinum and SnO2, catalysts prepared using SCFD exhibited higher activity than those prepared via wet impregnation, even at temperatures approximately 75°C lower. These literature examples were chosen because of the support material; supercritical fluid deposition is well-known to deposit dispersed particles on a variety of substrates.89,95
These studies demonstrate that supercritical fluids are capable of synthesizing a metal oxide supported catalyst with properties advantageous for reactions. Efficient use of materials will reduce energy costs for all phases of the life cycle. Strong adhesion of the expensive metals to the support will extend the life of the catalyst, reducing waste. Whether or not these benefits outweigh initial investment in the process itself are dependent on the catalytic application. As a case study for the life cycle assessment, and keeping in line with the general “green” theme of this research, the reaction of interest studied is the water-gas shift reaction, operating within a hypothetical automotive fuel cell system.
2.4 The Water-Gas Shift Reaction
Stringent emission controls and environmental standards, coupled with a general societal trend towards “green” energy and sustainability in the past 20 years, have led to a renewed interest in both environmentally friendly processes and fuels. Of the fuels, hydrogen is seen as a leading alternative to fossil-based fuels.20,96,97 Due to safety concerns involving hydrogen storage and its low volumetric energy density, on-board or localized, small-scale hydrogen generation is viewed as a possible solution. A typical liquid fuel processing system for a fuel cell and its relationship with the synthesis phase of the life cycle is shown in Figure 6.96
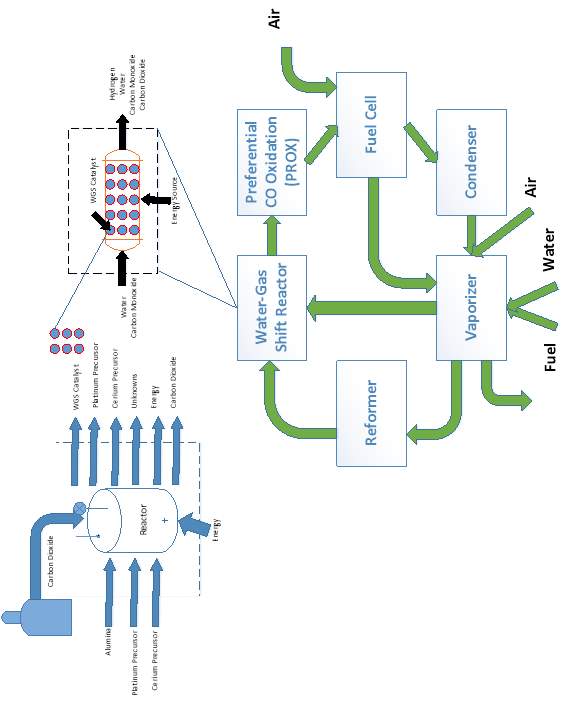
Figure 6: Standard fuel processing system coupled with an SCF catalyst synthesis process
The use of supercritical fluid deposition to produce water-gas shift catalysts, specifically combinations of platinum and/or ceria on alumina, has been previously performed in our laboratory.92 Although other catalysts formulations exist for this reaction, combinations of platinum and reducible metal oxides show the most promise and are more studied.98 A pseudo performance baseline can therefore be established for SCFD prepared catalysts.
2.4.1 Current Lab-Scale Performance of Pt-CeOx/Al2O3 WGSR Catalysts
Utilizing a holistic mindset for this research, the use phase of the product (catalyst) was thoroughly studied. The previous sections show that high implementation costs of supercritical technology can be alleviated if the output product displays superior performance.99 Recent published work using data collected in-house produced the following table (Table 1), comparing the performance of our in-house catalyst prepared by SCFD (final row) to those found in literature and prepared by other methods.92
Table 4: Comparison of WGSR catalysts
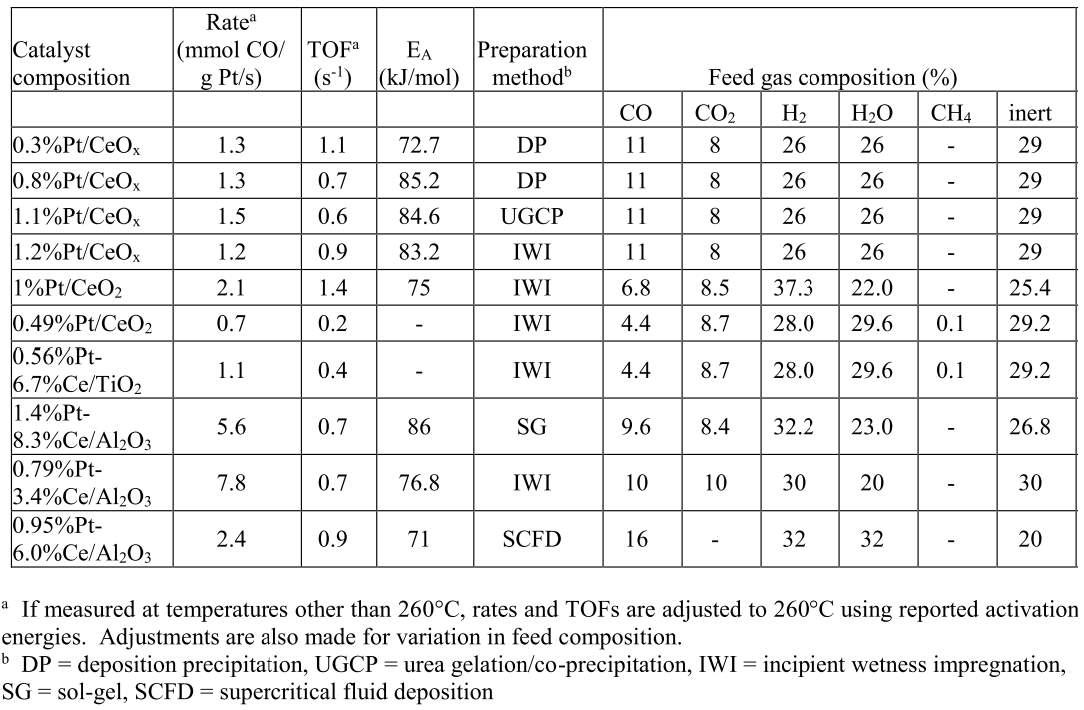
There is a large degree of variation in catalyst composition and feed gas composition in the table. The turn over frequencies are adjusted to reflect the variation in catalyst and feed gas composition, based off a proposed rate law reported in the literature and the platinum content. The SCFD catalyst exhibits the third highest overall performance (2.4 mmol CO/g*Pt*s) but also surpasses other preparations in its turn over frequency.100 These results demonstrate that SCFD is competitive with other synthesis methods from a product performance perspective. From economic and environmental viewpoints, it also shows the efficiency of SCFD in regards to material use and a minimization of post-synthesis solvent waste.
2.5 Conclusions
The literature shows a definite void exists regarding a true establishment of the environmental benignity of supercritical fluid deposition. No life cycle assessment has been performed for a Pt-CeOx/Al2O3 catalyst prepared using supercritical fluid deposition. Furthermore, there is scarce information regarding the environmental impacts of supercritical fluid deposition. The ability of SCFD to produce metal-oxide supported catalysts is recognized and catalysts prepared by SCFD exhibit high performance relative to other conventional methods. Additionally, supercritical carbon dioxide as a solvent is already industrially implemented, exhibiting high product recovery and little waste. These observations justify and support this research.
CHAPTER III
APPROACH & METHODOLOGY
Before beginning the life cycle assessment, the life cycle inventory must be completed. Accurate information is necessary and any assumptions made must be clearly stated and justified. Lack of data requiring assumptions and data uncertainty has led LCA studies to be deemed unreliable at times, but the life cycle assessment framework is generally accepted as a reliable decision making tool.38,101 The life cycle inventory includes all inputs and outputs for materials, energy, and any emissions/waste. Because the results of a life cycle assessment can vary based on what system boundaries are chosen,102 a clear and reasonable delineation must be made between the environment and the system life cycle. Furthermore, to properly quantify the environmental impact, a functional unit must be defined as a basis for calculations and comparisons. The work in this chapter includes the documentation of all necessary starting/exiting materials and calculation of the energy requirements for the synthesis phase of the system life cycle. To properly evaluate energy mixes/sources, a designated region of operation is chosen. Additionally, because this is a cradle-to-grave study, an assessment of the product’s performance for a system of interest is presented. Example life cycle assessment studies performed with SimaPro and their associated output are provided as an example of the final research deliverable.
3.1 Life Cycle Assessment Phase Descriptions and Data Collection
The boundaries for the life cycle of a product are relatively self-explanatory. Raw material extraction, processing of the materials, manufacturing, the use of the material, and finally its disposal are the phases that a product will go through in its lifetime. For this research, the challenge is not finding data concerning the acquisition of materials, their processing, or their recycling. The energy costs, along with the environmental impact, are documented and quantified for material acquisition, processing, and disposal phases. However, due to the novelty of the synthesis method, in-house data are required to complete the life cycle inventory. Furthermore, use phase data requires a scenario of operation for comparison with other, traditionally prepared catalysts.
3.1.1 Raw Material Extraction & Processing
Data that concerns the extraction and processing of raw materials did not deviate largely from what is contained in the database. The main area examined were the materials called for by the synthesis process. Switching to a new synthesis method involves the use of a different solvent, requiring the use of a different platinum precursor. For the SCFD pathway, platinum acetylacetonate was used. The alternative method, incipient wetness impregnation (IWI), required a platinum salt. Both pathways use -alumina as the support. Processing of raw materials did not differ. The source for CO2 was assumed to come from an already established process where carbon dioxide is an unwanted byproduct, generated from the combustion of carbonaceous fuels or from ammonia production. Carbon dioxide is essentially “free” in this regard as only 50% of industry generated CO2 is recovered.103 Manufacture of the catalyst at the CO2 source will reduce emissions from rail or road transport as well and reduce the expensive transportation of CO2. For this reason, no transportation processes included for CO2. When comparing SCFD to other catalyst preparation methods, material extraction and processing will not impact the overall conclusions due to the similar composition of both catalysts. It is important to note that infrastructure and capital equipment are neglected for this study as it has been to shown to be a minor contribution to the total environmental burden over a system’s life cycle.104
3.1.2 Manufacturing/Synthesis
For any life cycle assessment, a functional unit is defined to establish a criterion for comparison of systems. For this study, 1 kilogram of a catalyst composed of platinum and ceria supported on alumina is defined as the functional unit and labeled as Pt-CeOx/Al2O3. The final elemental composition represents a normal range found in the literature. This composition does not necessarily reflect the optimal formulation but serves as the basis for the life cycle assessment. This functional unit was also chosen for ease of calculations in the life cycle assessment software.
There is a scarcity of LCA data available concerning supercritical fluid deposition. Much of the data required was collected within the lab. Figure 7 shows the reactor used for lab-scale synthesis from supercritical CO2.
Figure 7: Lab-scale SCFD catalyst manufacturing vessel
The life cycle inventory for user-defined materials can be found in the appendices. The materials chosen for the synthesis are based on their solubility in carbon dioxide.90 This is an important aspect of solvent selection and certain precursors are easier and/or cheaper to manufacture. The concentrations of the reactants were chosen for comparison with those in the literature and do not necessarily represent the optimal ratio for catalytic performance. Previous in-house work has demonstrated that the deposition occurs with a ≈ 70% efficiency; however, this could be from non-optimal reactor conditions and other synthesis factors. Therefore, it was assumed that the reaction went to 95% completion in a larger scale operation. Calculation of the energy requirements required to bring the solvent to supercritical conditions were performed using REFPROP with a pump efficiency of 65%.105 It can be assumed from the low concentrations of the input materials that all the energy supplied to the reactor goes towards heating of the CO2 solvent. No post-synthesis processes are included aside from drying of the pellets. The detailed experimental procedure for the preparation of the catalyst, both from SCFD and IWI, can be found in the appendices.92
3.1.3 Use Phase Description
The switch to SCFD as an alternative synthesis route is not strictly for environmental reasons. There are advantages in the product that SCFD generates over those from traditional methods, specifically a high dispersion and homogeneity.106 Literature values for sol-gel and incipient wetness impregnation of Pt on ceria and/or alumina range anywhere from 5% to 69%, highly dependent on metal loading and post deposition processes.107–109 High dispersion of the metal on the metal oxide typically results in smaller average particle sizes, which increases the performance/conversion and provides optimal material usage. For the use phase, there are two performance metrics of interest: the reaction rate per mass platinum, and the time on stream, which is essentially a measure of the durability of the catalyst. For comparison and to ensure appreciable activity, catalyst performance is measured at 300°C. An appropriate amount of catalyst was loaded, approximately 0.3 grams. Realistically, this temperature is acceptable for a fuel cell system that makes use of hydrogen from a reformate stream or that relies on methanol for hydrogen generation. The economic and environmental viability of the fuel cell system itself is not within the scope of this research, but an operability scenario is required to properly compare the competing products and complete the life cycle assessment.
The performance of an automobile using a hydrogen fuel cell system should be approximately equal to that of current gasoline fueled automobiles. For simplicity, 250 miles as an average distance the vehicle should travel is reasonable, which equates to 4 kg of hydrogen.110 Calculations can also be based on the fuel cell power output, making a reasonable assumption of 50 kW (small car) for the fuel cell automotive system.111 The basis for calculations in this study is 50 kW.
Fuel cell operation requires pure hydrogen, but safety concerns and storage pose limitations; however, onboard hydrogen generation is a solution. Due to current infrastructure, natural gas is considered one of the top sources of hydrogen, although gasoline could function as a hydrogen source too. The use of hydrocarbons also presents difficulties with carbon monoxide being a fuel cell poison. The typical CO allowance is specified at 10 – 50 ppm.107,112 This requires a cleanup step in the process (water-gas shift reactor followed by preferential oxidation), with a typical CO concentration exiting the WGSR of 1% by volume.113 Subsequently, there are two key requirements derived:
- The outlet from the water-gas shift reactor shall produce a minimum of 50 kW of power.
- The outlet from the water-gas shift reactor shall contain a maximum of 1% carbon monoxide by volume.
From these specifications, the system boundary was drawn (segmented line) and is shown below. Molecules are listed to show material flow.
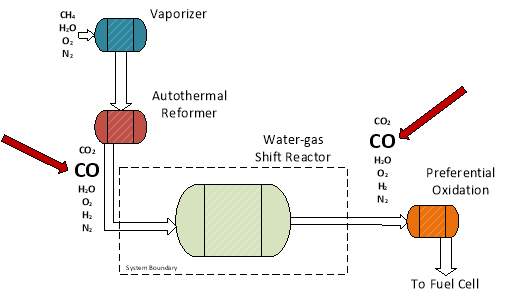
Figure 8: System boundary for catalyst performance testing
The efficiency of the fuel cell is assumed to be 50%, requiring a hydrogen flow rate of ≥ 0.84 grams/second. Calculations for the required methane feed rate are based on a paper by Ayabe et al.114 and can be found along with additional assumptions in Appendix A. From the requirements, the only concern is making sure that the carbon monoxide level is reduced to ≤ 1% by volume. An interesting question arose from the calculations: is it better to generate the necessary hydrogen strictly from the reforming step or reduce the performance of the reforming step, allowing the WGSR to make up the difference in hydrogen generation? It was concluded that the performance of the reformer and the preferential oxidation step is outside the scope of the scenario, and the environmental burden of the fuel cell system is limited strictly to the WGSR catalyst and no other associated emissions. The requirements outlined are therefore restricted to conversion and performance metrics. Furthermore, the feed to the water-gas shift reactor contains reducing gases which affect metal dispersion, requiring a “catalyst lifetime” or “robustness” be specified as well.
“Time on stream” is a measure of the catalyst’s degradation over time as coalescence of the metals reduces performance. Mitigation and elucidation of sintering mechanisms is of major interest in the catalysis community. It is difficult to extrapolate and predict the longevity of nanoparticles due to complex coalescence mechanisms. Work has been done in an attempt to understand the sintering kinetics of nanoscale platinum particles through the use of electron microscopy.115 Hansen et al. studied the sintering of palladium nanoparticles on silica, concluding that the majority of sintering occurs from atomic movement, rather than particle migration.7 Because SCFD produces extremely small particles, some of them atomically dispersed, addressing the performance over time is mandatory. Industrially and in real world applications, the problem is demonstrated as well. For example, a study by General Motors shows how the dispersion of the metal decreases through the life of a catalytic converter.60

Figure 9: Loss of exposed metal over time in a commercial automotive catalyst60
Within 40,000 miles, the dispersion has dropped around 90%. While it is true that emission standards are still being achieved, a large portion of the initial material costs could be saved if the metal was more resistant to thermal and kinetic forces. Raising the temperature of the reactor results in an increase in the rate of reaction, simultaneously increasing the likelihood that particle agglomeration will occur and reduce the dispersion and performance of the catalyst. The same reaction performance can be achieved at a lower temperature if the number of active sites is higher. For this reason, the reaction rate per mass of platinum is an excellent method for quantifying performance. Time on stream studies are fairly common in the literature for catalysts similar the ones in this study,111,116 however, there have been no studies regarding stability on those produced from supercritical CO2, let alone under the same gas composition. Validating the catalyst over the same range and time scale of the General Motors study is impractical for this work, and inappropriate because of the high operating temperature experienced by catalytic converters, normally >=650°C. The operability scenario involves a fuel cell vehicle operating under reformer conditions, the composition of the feed determined from the autothermal reforming of methane; therefore, the temperatures experienced are much milder. During vehicle use, there are kinetic considerations to account for in addition to thermal effects. A reasonable assumption can be made that the thermal forces will have a much larger impact on metal dispersion compared to any kinetic forces due to the structure of the catalyst. The temperature of the reactor will therefore play the largest role in the lifetime of the catalyst, influenced as well by the composition of the feed to the reactor. A study by Phatak et al. from 2007 varied the temperature from ≈ 250°C to ≈ 320°C to investigate the performance of Pt/Al2O3 catalysts operating under reformer conditions for fuel cell applications.108 These operating temperatures are consistent with those found in Table 3. It was assumed that heat integration within the fuel cell system allows the entering feed to the water-gas shift reactor not to fluctuate substantially from the desired operating temperature of 300°C. It was important in this instance to look to the principles of green engineering for guidance. It is recommended not to design for “immortality”, and also not to design for unnecessary performance. Subsequently, testing at abnormally high temperatures and harsh conditions is unnecessary due to the small likelihood of their occurrence. Assessment of the stability of the catalyst is performed at a variety of temperatures > 600°C to ensure degradation and the time was recorded for each measurement. The longevity is based on the loss of catalytic activity over this time period, producing a rate of dispersion loss (agglomeration) per unit time. Through the use of the Arrhenius equation, an activation energy and pre-exponential factor are calculated to estimate the average catalyst life-time at 300°C.
3.1.4 Regeneration, Recycling, and Reuse
The expensive and rare materials, mainly platinum, that make up the catalyst are designated as essential for recovery. With environmental pressure and increasing PGM demand, particularly in developing nations, reclamation is essential to meet future needs. Because of the catalyst’s three-component composition, it is relatively simple to recycle and feed back into the manufacturing phase. This is a safe assumption to make as the PGM (platinum group metals) recycling industry is well-established.117 There is no additional investment needed as the industry is equipped to deal with the catalyst compositions; however, this also assumes that the material flow rate of aged/spent catalysts is large enough to meet the production demand of new catalysts.
Aside from the costs, recovery of platinum instead of mining new material will lower the environmental impact significantly. This is true for the majority of materials; it is nearly always more energy intensive to mine and extract virgin materials than it is to recycle them. The creation of new mines, depletion of current reserves, and the additional need for PGM/ore separation all contribute greatly to environmental burden. This goes for alumina as well, where as much as 16 MJ are needed to produce 1 kilogram.118
Resistance to deactivation data collected from “use” phase testing is essential for describing the best end of life path the catalyst should take. Unless the dispersed platinum can be stabilized on the surface, regeneration and reuse of the product is deemed unlikely due to agglomeration and loss of activity. However, it remains a possibility to use the catalyst for a different function in the future, perhaps in a different reaction. Although unlikely at this point in time, the assessment could still be performed if these options are available in the future, possibly through a detailed understanding of the surface chemistry that occurs during the synthesis phase.
3.2 Life Cycle Assessment of Supercritical Fluid Deposition
The in-house data is aggregated and used to complete the input for the life cycle assessment. SimaPro is used to complete the analysis. ReCiPe 2016 is used as the impact assessment method and the ecoinvent database contained within SimaPro provides inventory data that is not collected in-house or from literature. To gauge the environmental burden of SCFD in relation to other conventional synthesis pathways, sets of catalysts prepared from conventional methods are investigated. The diagram below shows the pathway for the processes being compared and their damage assessment categories.
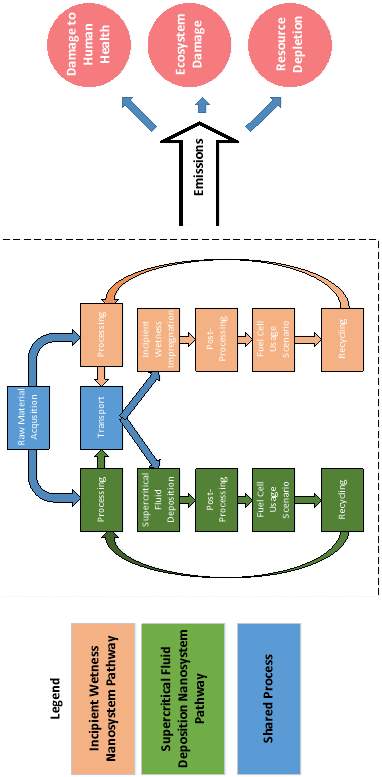
Figure 10: System boundary of proposed study for competing catalyst preparation methods
Some processes are shared because there is no difference in the emissions. In the event that a database value is unavailable to obtain the necessary information to complete the life cycle inventory, the literature is consulted or experiment performed.
3.2.1 Handling of Uncertainties & Sensitivity Analysis
The uncertainty in a life cycle assessment is inherently a challenge. Some of the data within the ecoinvent database is an average supplied from a single source or industry expert. There occasionally is no uncertainty associated with the mean value. The ecoinvent database uses a mix of calculated and qualitative estimates to gauge uncertainty in data. The reasoning behind the calculations of uncertainties can be found in the ecoinvent methodology report.41 When using ReCiPe, the uncertainty in the results depends on the cultural perspective used: hierarchist, egalitarian, or individualist. Each perspective comes with different assumptions for characterization and relationships between the life cycle inventory and midpoint/endpoint indicators. For this research, due to the number of variables being manipulated, a single cultural perspective is implemented. The hierarchist cultural perspective is chosen because of its balance in weighting environmental/societal needs and its wide use in the scientific community.44 For comparison of the damage metrics between the two production pathways, Monte Carlo simulations were performed with a stop criteria of standard error of mean being equal to .005 (99% confidence interval).
Implementation of the catalyst in a system requires looking at the possible range of operating conditions it will encounter. This could be anything from a change in the feed composition to an increase and/or decrease in the reactor temperature. These operating conditions influence the lifetime of the catalyst, and thusly, have an effect on the life cycle assessment. Feed conditions were kept at the calculated ratios available in the appendices.
Looking at the life cycle of the catalyst overall, several scenarios were investigated for sensitivity analysis, as the selection of a single process can drastically alter the conclusions of the assessment. The key areas or “hot spots” to focus on include:
- Platinum content in the nanosystem
- Recovery of energy in the supercritical fluid deposition system
Region of operation can affect results due to different material production and extraction methods. For example, crude oil from Canadian oil sands results in 5 times greater greenhouse gas emissions than obtaining crude oil from Saudi Arabia/Iraq, a scenario where LCA results can be substantially affected.26 However, as environmental damages are a worldwide concern, average global production and associated source-region of materials are assumed when available. For user-defined materials, United States averages are used. Because this research focuses on reduction in platinum use, only platinum was assumed to be recycled with a rate of 95% recovery. Furthermore, the overall elemental composition of the catalyst and with the needed infrastructure already in place, the recycling is assumed to occur in a spent catalytic converter precious metal recovery facility.
3.2.2 Accelerated Life Cycle Testing
Strongly anchoring metals to the surface of the support is key in the design of precious metal water-gas shift catalysts. This robust surface bond enables particles to resist sintering, facilitating high catalytic activity and a longer operational lifetime. The temperature, feed composition, and dispersion of the metal all play a role in the performance of the catalyst, with complicated relationships.
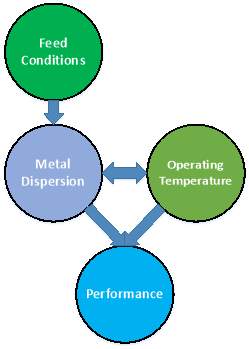
Figure 11: Influence of feed composition, dispersion, and operating temperature on performance
Due to the practicality of running the catalyst for an extended time, shorter run times at higher than the operating temperature (300°C) are used in what is essentially highly accelerated life cycle testing (HALT). These results are used to estimate the stability of the catalyst at normal operating conditions, and to extrapolate and gauge the lifetime of the catalysts. Nucleation was assumed to be the dominating factor in product degradation.
3.3 Conclusions
3.3.1 Hypothesis Validation
Every process has an associated uncertainty with the emissions that it produces. The ecoinvent database includes uncertainties and uncertainty distributions for the majority of process data. SimaPro is a powerful software and can repeatedly sample from each process’ distribution to ensure proper conclusions are drawn from a life cycle assessment. To properly compare the two product pathways, Monte Carlo simulations are performed to validate the impact assessment. For comparison, these scenarios are applied to two separate catalysts. This results in a Pt-CeOx/Al2O3 catalyst prepared by two production pathways:
- Incipient wetness impregnation of both platinum and cerium precursors
- Supercritical fluid deposition of platinum precursor only, IWI of cerium precursor
The hierarchist cultural perspective is used to define assumptions and normalization of the damages is performed for ease of comparison. The hypothesis was tested for two product life cycle phases:
- Product development phase
- Use phase
Validation is provided by a Monte Carlo simulation of the entire product life cycle. To say that supercritical fluid deposition is a “greener” alternative requires that the SCFD catalyst surpass the traditional method in all environmental damage categories, as a consensus on the importance of each category is undefined and left to interpretation.
3.3.2 Summary
Going through the literature, there are few studies that address products throughout their entire life cycle, let alone studies that model the situation in this research. The vast majority of the studies encountered investigate the production of a simple solvent or product, such as hydrogen. All hydrogen behaves the same, whereas all Pt-CeOx/Al2O3 catalysts have the potential to behave differently. Despite possessing the same composition, the properties at the surface, such as metal dispersion, particle size and geometry, and intermolecular forces, can drastically differ depending on the preparation method. In turn, these nanoscale features influence the performance of the catalyst is affected, causing differences in the environmental impact. This study addresses these differences, taking into account the overall product life cycle of a precious metal-based catalyst prepared by two competing methods, a unique attribute not found in other literature studies.
The research methodology includes a laboratory phase and a simulation phase. In order to properly address the environmental burden, a full characterization of both products is performed. This data includes an elemental analysis, particle size analysis, and performance data. The data collected are used as input for SimaPro to assess environmental burden. The work in this section includes the majority of the necessary life cycle inventory for the synthesis and use phase of the life cycle assessment, along with a scenario of operation. Calculations and further assumptions are attached in appendices. Figure 12 on the following page presents how the research is organized.
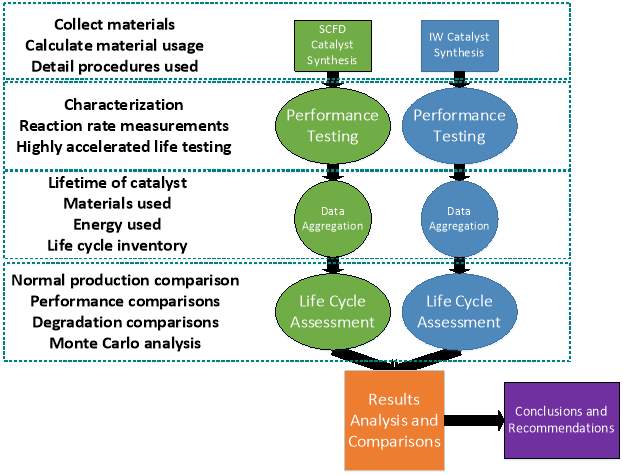
Figure 12: Research organization
This chapter establishes a framework and outline for future life cycle assessment work. In general, the research is an environmental analysis of the introduction of a new and novel manufacturing method for a catalyst product. The manufacturing method utilized is supercritical fluid deposition. However, the area of study is not limited to this phase only. Introduction of this new method requires different starting materials because of new process constraints. Also, the product will have arguably superior performance and different characteristics, requiring a study of the use phase as well. End of life concerns should not be a large factor due to existing precious metal recovery infrastructure. With literature data indicating that supercritical processes usually recoup energy losses through superior product performance, it is vital to perform the life cycle assessment for the entirety of the product or process life.
Upon completion of this study, a framework exists for the life cycle assessment of other catalyst compositions prepared from supercritical carbon dioxide. The materials chosen for this study are not the only components being investigated, and with the use of software, studies on multiple compositions is easily accomplishable if performance and inventory data are available. The interrelationship of the emissions from a products life cycle and the cumulative energy demand of its production make an economic analysis a future avenue of study as well.
The work presented in this chapter demonstrates the unique nature of this research. Understanding the chemistry that occurs at the nanoscale allows guided and thoughtful optimization. Once the process is understood, how it influences the life cycle of the product as a whole can be determined. Life cycle assessment provides a utility to see how the manipulation of materials at the nanoscale propagates to the macroscopic level, where ramifications as large as climate change can be studied. This research exemplifies the need for a systems thinking approach in rational catalyst and chemical process design.
CHAPTER IV
ANALYSIS & EXPERIMENT
This chapter is compartmentalized into three sections with the groupings aligning with the simulation layout provided in SimaPro. Raw material extraction, processing, and manufacturing constitute one segment, known in SimaPro as an “assembly”. From a systems development life cycle view, this constitutes every phase up to and including development. To identify the important areas for optimization and for general intrigue, platinum content and energy recovery in the supercritical product pathway are varied to assess their contribution to the overall environmental burden of the product development life cycle. The use phase and end of life phase (testing and disposition) constitute the remaining two. Each section contains the life cycle inventory and any information obtained from laboratory experiment for both production pathways. Furthermore, it includes the damage assessment of each pathway. The final section contains the overall results of the entire life cycle for each nanosystem production pathway. General trends in reduction of platinum content and energy recovery in the SCFD production pathway are also presented.
4.1 Raw Material Extraction, Processing, and Manufacturing
The boundary of analysis in a life cycle assessment must be properly drawn for any meaningful analysis to occur. Possibly due to the relatively new nature of life cycle assessment, there was inadequate life cycle data in the literature concerning industrial preparation of metal acetylacetonates and metal salts. Therefore, an estimate was made for both platinum precursors along with the cerium precursor. Guidelines proposed by the ecoinvent methodology were followed to estimate the life cycle inventory for materials not found in the econvent database.119 Many papers reference a 2015 environmental report from Gendorf industrial park,39,119 a multi-commodity production facility in Bavaria; however, it was decided to use data based on United States production capabilities. Each reaction was assumed to operate at 95% efficiency. When energy use for the production of a chemical could not be found, an average energy consumption per kg of material was based on United States energy consumption data provided by Kim and Overcash.120 The average calculated was in agreement with the Gendorf industrial park figure. Transportation distances were assumed to be the average United States mix of truck and rail. These values were obtained from the 2012 Commodity Flow Survey published by the Bureau of Transportation Statistics and U.S. Census Bureau.121 Rail transportation accounted for the initial transport and truck was used for final delivery with the average distance for the “Basic Chemical” commodity used for all materials. Average water consumption was based on a 2002 figure from The Dow Chemical Company and was similar to the Gendorf industrial park value as well.122 All materials that were not created for this study follow a global average in regards to production emissions and transportation values provided in the ecoinvent database. Production of the cerium salt was assumed to be from dissolution of bastnasite ore in hydrochloric acid. The source of the ore was a global average, with a typical concentration of 50 wt % cerium oxide. The carbon dioxide used was assumed to be released to the environment at the point of production of the catalyst. For ease of calculation, it was assumed that the excess water used for dissolution of the metal salt was equal to the water emitted from the process. During the manufacture of the catalyst from supercritical CO2, it is important to understand the interaction at the interface between the platinum precursor and the alumina surface. In order to dissolve in carbon dioxide, platinum must be bound to another component, in this case, acetylacetone. Essentially, acetylacetone “masks” the chemical properties of platinum in order for CO2 to dissolve the metal. Upon contact with the alumina surface, the platinum precursor reacts and deposits on the alumina surface. Preliminary experiments in the lab have found that acetylacetone leaves the surface upon depressurization of the reactor. *insert appendices* Acetylacetone, or 2,4-pentanedione, is known to be a toxic emission, although some studies suggest it is biodegradable.123 Regardless, from experiment, it is proper to include acetylacetone as an emission during the supercritical CO2 synthesis pathway. The table below contains all user-defined materials for this study and important assumptions.
Table 5: User-defined materials and important assumptions for this study
| User-defined materials | Assumptions |
| Acetylacetone | 2.84 grams of water per gram of product122 |
| Cerium Chloride | 80% of water derived from river source
20% from well source124 |
| Isopropenyl Acetate | 95% reaction efficiency119 |
| Platinum Acetylacetonate | 99.8% of leftover reactants to water
0.2% to air119 |
| Hexachloroplatinic Acid | 217 km via truck
1332 km via rail121 |
| Sodium Acetate | Average US energy use and
energy source for material production120 |
Expectations are that the main disadvantage to switching to a supercritical deposition system is the increased energy expenditure. However, switching to a different non-traditional preparation method requires a different metal precursor due to different process conditions, i.e. the use of carbon dioxide as a solvent. The metal precursor in IWI requires further processing to be utilized in the SCF system. The addition of another process inherently leads to greater environmental impact in the life cycle chain overall. Below we see the effects of the additional process with a comparison between the production of the IWI precursor and the SCFD precursor, platinum chloride and platinum acetylacetonate, respectively.
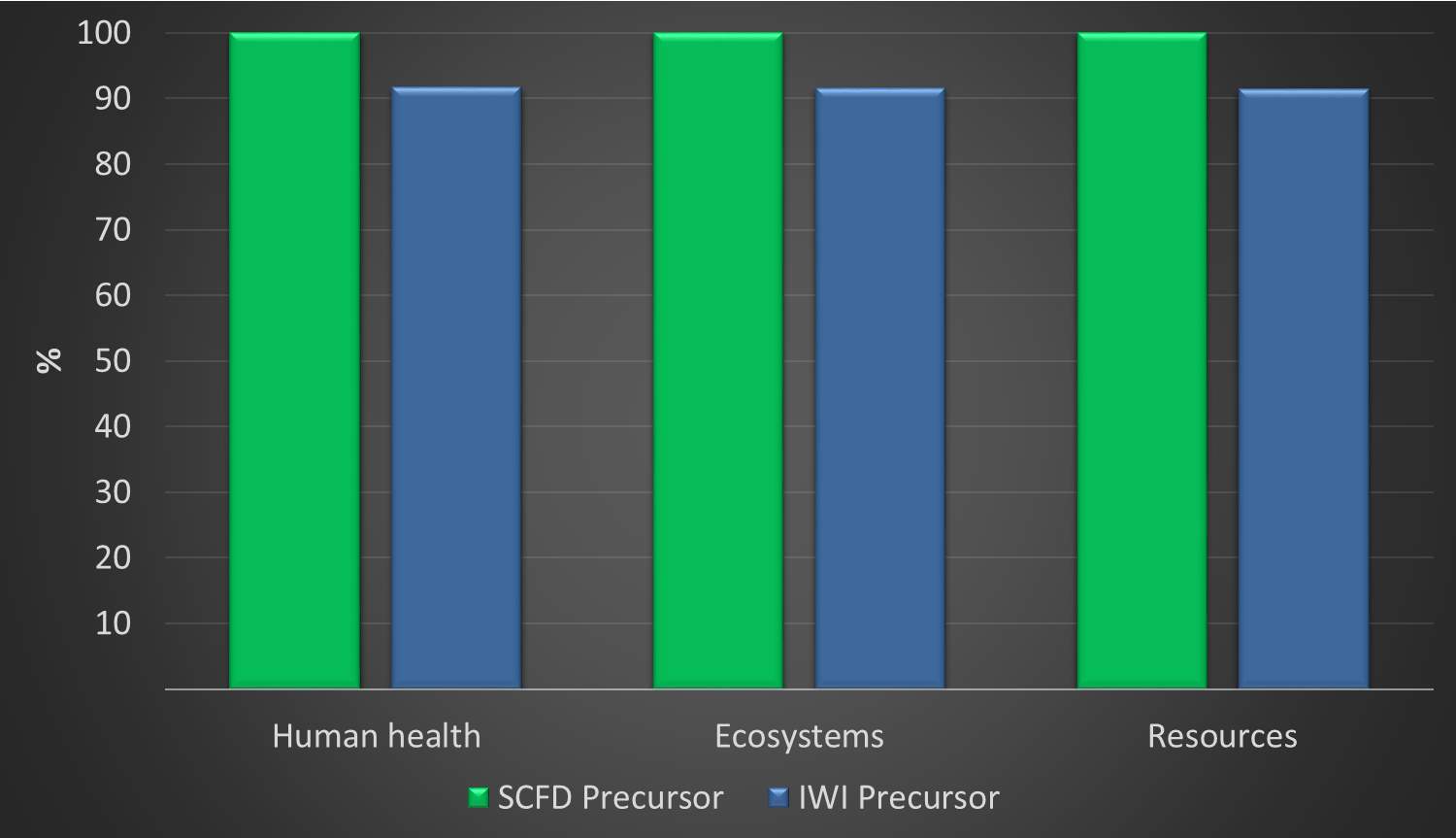
Figure 13: Comparison of Product Development Phases
The platinum salt, hexachloroplatinic acid, is transformed into the organometallic precursor, platinum acetylacetonate, used in the SCFD process. The difference in the two can be traced to the production of acetylacetone, the additional water consumption, and additional transport requirements, and additional energy requirements. This difference is relatively small and assumes the platinum salt is the precursor used in the production of platinum acetylacetonate. If this is incorrect, the two platinum precursors may be closer than what this result would suggest.
It was found that the mining process and the tailings swamp the results of the LCA for the manufacturing phase of both production paths. Figure 14 demonstrates this, showing the contribution of each process to the total DALY value of the SCFD product development phase. Results were similar for the IWI product pathway.
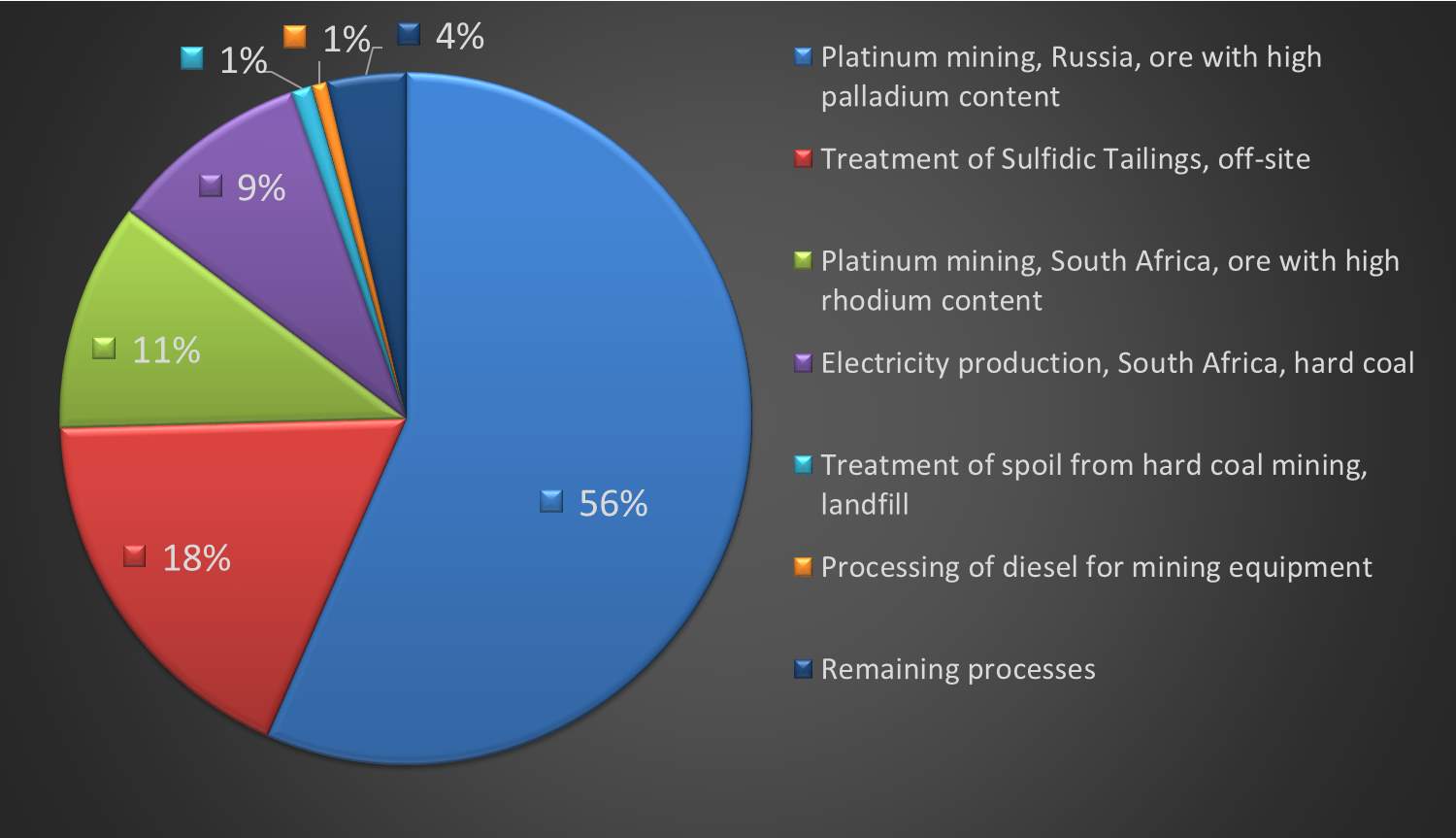
Figure 14: Contribution of platinum related processes to human health (DALY)
Both ecosystem and resource damage categories resulted in similar results. Looking at the source for the processes in Figure 14, they can all be traced back to the platinum mining process, with all associated processes combining to make up nearly 100% (99.7%) of the total burden for all damage categories. It’s important to note the different regional sources of platinum in this figure, those being Russia and South Africa, and their different contributions to the environmental burden. The difference in their relative environmental contributions is significant and highlights the reasoning behind using global averages for this study. Despite making up approximately one percent of the product, the addition of platinum to the catalyst nanosystem dwarfs all other materials in terms of environmental burden. This is mainly from intense energy use involved in raw material extraction coupled with the treatment of harmful sulfidic tailings, a large problem in the mining industry.125 Furthermore, the source for this energy is all fossil fuel derived (coal, natural gas, petroleum). These fuels produce emissions that significantly impact all damage metrics.
To get a better understanding of each material’s contribution, platinum was removed from the product assembly to investigate other sources of environmental burden.
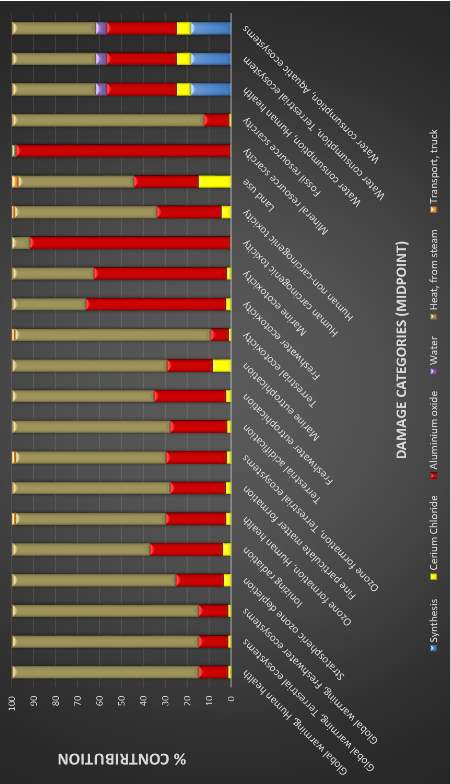
Figure 15: Environmental burden with platinum content = 0, midpoint damage categories
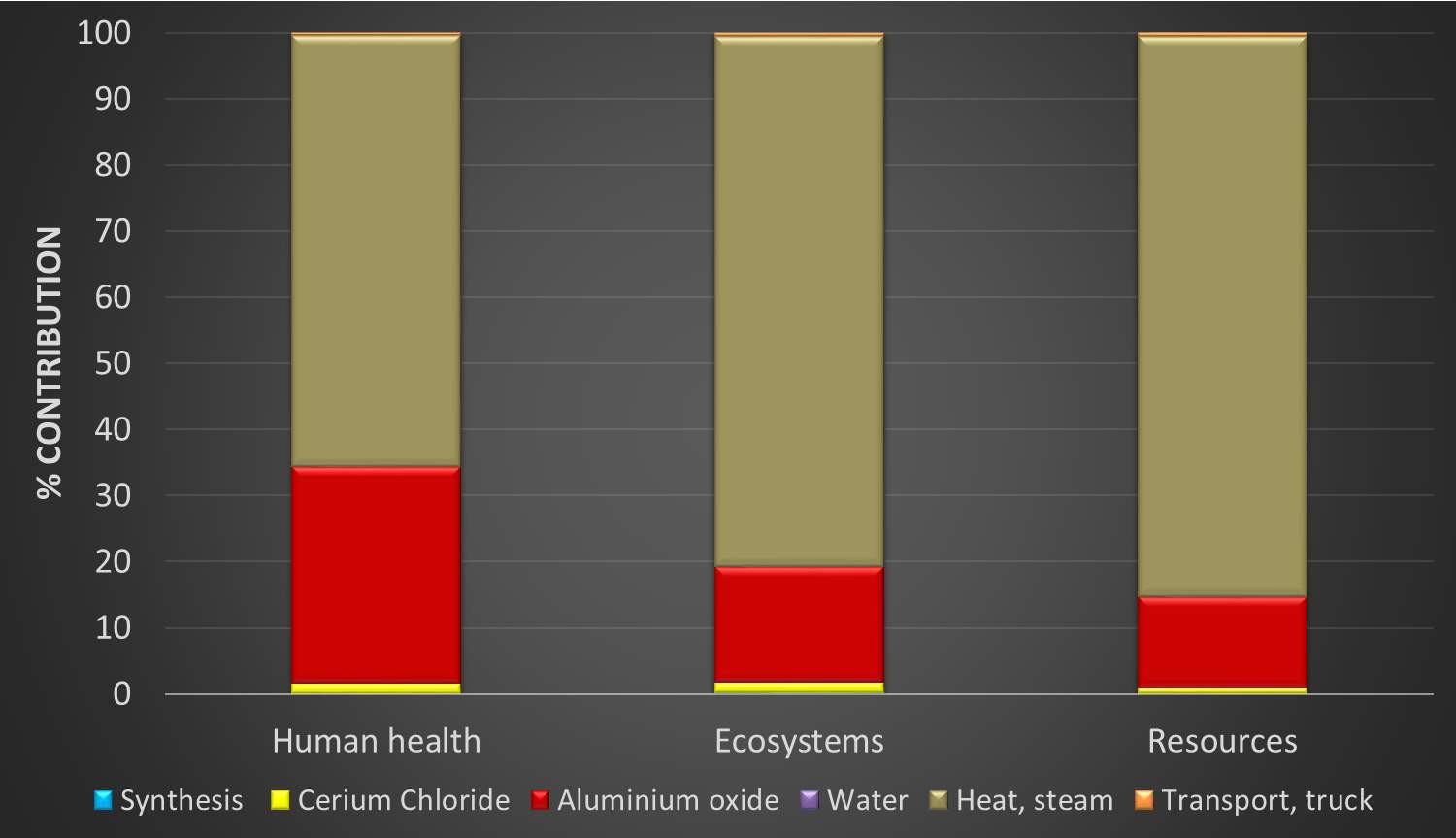
Figure 16: Environmental burden with platinum content = 0, endpoint damage categories
The analysis indicates that generation of energy is the next dominant contributor to the environmental burden. The energy use in the supercritical process is substantial, defined in this study as 65 MJ and contributes 66% of the total when platinum is removed from the catalyst formulation. This energy requirement is calculated from the carbon dioxide necessary to dissolve the platinum precursor based on solubility constraints.
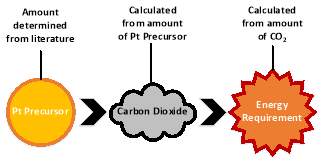
Figure 17: Calculation of SCFD Reactor Energy
This energy requirement can be reduced, either through an increased understanding of the chemistry itself or energy recovery. The midpoint category, “fossil resource scarcity”, indicates that the source of the energy is fossil fuel derived, and is ultimately traced to natural gas. Aluminum oxide, or alumina, is the next largest contribution, a reasonable result as it makes up 99% of the final nanosystem.
The assumption of the reaction mechanism in the supercritical process results in what is most likely the upper limit in regard to energy consumption. It assumes that the total amount of carbon dioxide necessary, and consequently the energy requirement, is linear with the solubility limit of the platinum precursor. This may not be true and further study is required. Regardless, these two areas can be considered “hot spots” with platinum being the parameter of greatest concern. Reduction in platinum is the most promising path for reducing environmental burden.
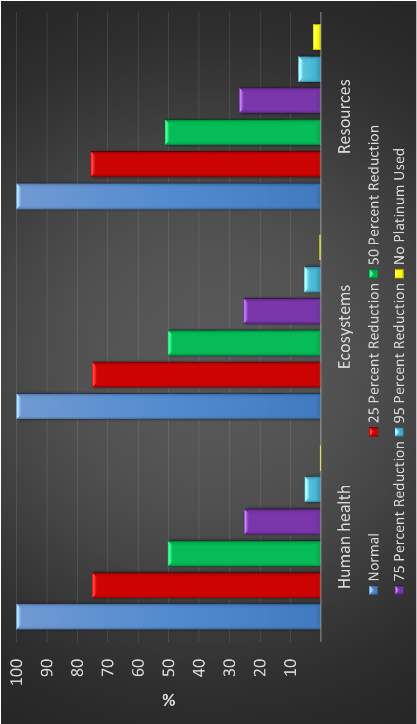
Figure 18: Effect of platinum reduction in the SCFD product development phase
This highlights the importance in metal dispersion in systems that make use of precious metals. Each damage metric improves in a linear fashion. Every 25% reduction in platinum use results in an approximate 25% improvement in environmental burden. With no platinum being used, damages are reduced across the board by over 90%. Resource depletion shows the smallest benefit in platinum reduction. This is traced to the large amount of alumina in the catalyst.
Supported by this research, reduction in metal content through the exploitation of nanotechnology is a feasible solution. It is a realistic way to reduce the burden and for once, environmental concern is in agreement with economics. The average price for platinum in 2012 was $50 per gram.126 The high price of platinum in combination with the burden leaves room for incorporation of stabilization metals as well. Incorporation of metal oxides, such as the cerium in this study, can stabilize the dispersion of the metal and promote activity.127 Optimizing platinum use through process improvements and/or material synergy are promising avenues.
The automobile industry still incorporates PGM (platinum group metals) into catalytic converters, electric-only cars being the exception. 40% of global platinum consumption is by the automobile industry. For this reason, substitution of platinum, at least at this point in time, seems unlikely. If the platinum content cannot be reduced or replaced with another component, the possibility for energy recovery is the next parameter for investigation. Figure 18 models the product development with platinum removed from the nanosystem to better illustrate the impact of energy recovery in a supercritical process.
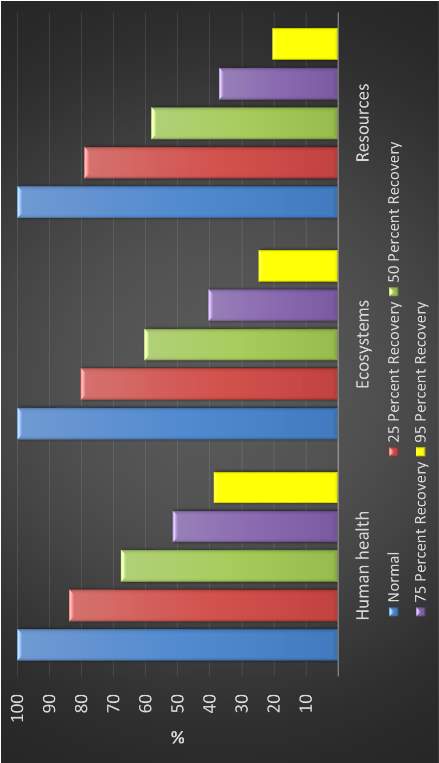
Figure 19: Effect of energy recovery in SCFD product development (no platinum)
Both energy recovery and platinum reduction are possible ways to reduce the burden of the supercritical process. The high pressure of the supercritical process makes energy recovery viable. A study by Calzavara et al. looks at recovery of energy from supercritical water in a biomass gasification process.128 The critical conditions for water, P > 220 bar and T > 374°C, are much higher than those of carbon dioxide. Using a turbine to recover work energy is more likely than heat recovery due to the relatively mild operating temperature.
Comparison of Figure 17 and Figure 18 shows that platinum reduction is a better path for reducing damages, with a 25% reduction in platinum being approximately 10% better in reducing damages than reducing energy consumption by 25%. However, this is without the incorporation of platinum. The reintroduction of platinum into the nanosystem highlights the true, and miniscule, benefits of energy reduction in the supercritical process. This difference in environmental benefit is shown in Figure 19.
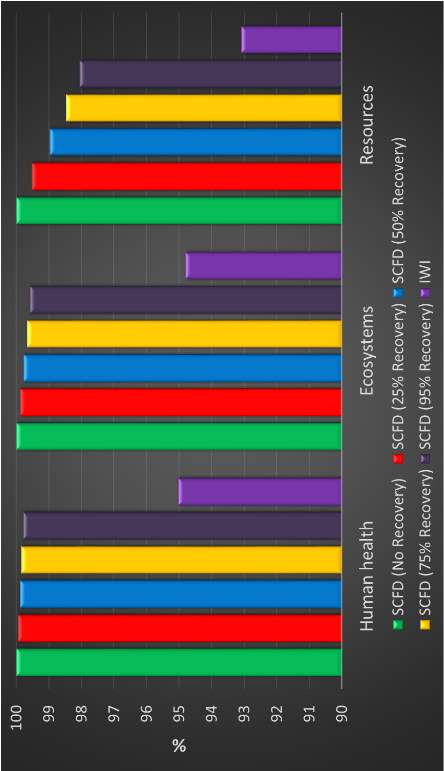
Figure 20: Recovery of energy in SCFD process w/ platinum
Overall, the energy required to achieve supercritical conditions is a minor contributor when platinum is re-introduced. The process conditions for IWI are at ambient conditions and most of the energy use is minimal. The energy use can be attributed mainly to filling, agitation, and draining of the reactor vessel. The deposition was assumed to occur at 95% efficiency. The SCFD system requires much more energy due to the critical parameters of carbon dioxide, 2000 psi (13.8 MPa) and 60°C (140°F). The carbon dioxide utilized for the deposition was assumed to come from a waste stream and therefore not accounted for in emissions or material input. Although capital equipment is not involved in this study, there would be additional control systems and costs associated with constructing a high-pressure system.
The large concern in literature with implementation of a supercritical process is the energy usage. Although there is no solvent evaporation step or drying step, the energy consumption for the supercritical process is well above that of the IWI method and is the 2nd highest contributor to environmental burden. Combined with the additional materials and energy required for a different platinum precursor, the SCFD production contributes 5% or greater in all three damage metrics, relative to the IWI process.
General trends were observed in initial “hot spot” analysis:
- The addition of platinum, in any capacity, greatly increases the environmental burden of the nanosystem
- Energy consumption is the 2nd largest contributor to environmental burden
- Reducing platinum use in the system, either through optimization or by replacement, decreases the environmental burden significantly
- Substitution of a different substrate or “carrier” material offers a reasonable method for reduction in environmental burden
- The additional energy to achieve supercritical conditions and in the manufacture of the SCFD platinum precursor contributes very little environmental burden
4.2 Use Phase
The decrease in the CO content to 1% by volume was the only requirement to be satisfied. Because of this, simulating the actual effluent from the autothermal reforming of methane is not a factor. The comparative nature of this study along with other constraints supports this decision. Furthermore, for comparison with literature catalysts, it is important to maintain a similar feed composition. As such, only carbon monoxide, water vapor, nitrogen, and hydrogen entered in the feed. Both catalysts were validated under the same conditions. The requirements defined in Chapter 3 for validation are:
- 1) The outlet from the water-gas shift reactor shall produce a minimum of 50 kW of power.
- 2) The outlet from the water-gas shift reactor shall contain a maximum of 1% carbon monoxide by volume.
The nature of these requirements being coupled dictated that requirement 2 be satisfied first, as requirement 1 can take any value above 50 kW. Furthermore, requirement 1 is satisfied before the catalyst is added to the system. Reducing carbon monoxide content is therefore the only requirement to validate. The graphs below compare the performance of the systems at the same operating conditions.
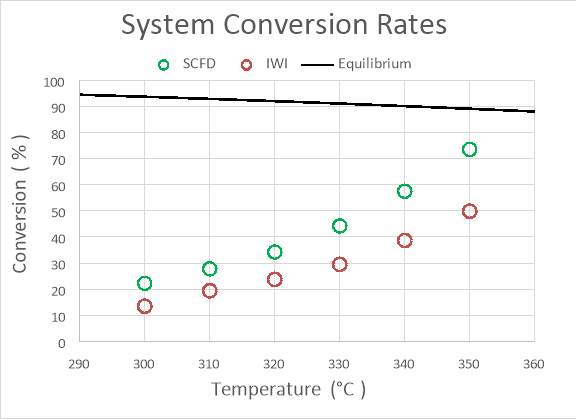
Figure 21: Performance of nanosystems from 300°C to 350°C
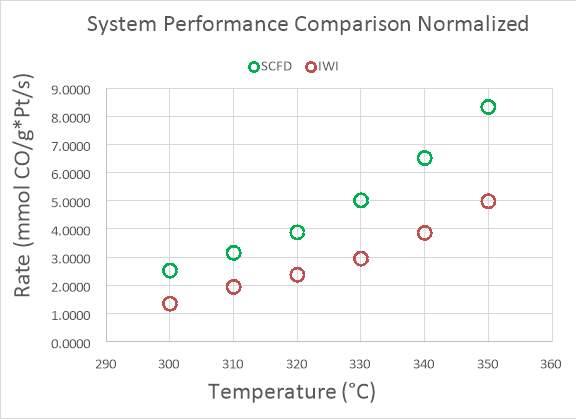
Figure 22: Normalized performance of nanosystems from 300°C to 350°C
The table below shows the data necessary to properly characterize the catalyst nanosystems. This data was used for life cycle assessment analysis.
Table 6: Nanosystem characterization data
| Production Path | System Composition (%) | Bed weight (g) | Dispersion | Performance at 300 °C (Conversion %) | Outlet CO Concentration (Vol %) | Ratio of Rate Constants (SCFD/IWI) at
300 °C |
|
| SCFD | Pt | 1.0 | 0.2872 | 0.4 | 22.2 | 12.3 | 2.01 |
| Ce | 4.7 | ||||||
| IWI | Pt | 1.1 | 0.2965 | 0.2 | 13.5 | 13.7 | |
| Ce | 5.3 | ||||||
The minimum catalyst required was estimated from the ratio of the rate constants for both catalysts at 300°C. The rate constant was calculated using the rate law proposed by Germani and Schuurman.94 Accompanying calculations can be found in the appendices. The system composition is similar with the difference in both platinum and cerium content not statistically significant. Post-manufacturing or synthesis processes were the same, with any difference in performance occurring due to the production path. The lower performance of the IWI catalyst is attributed to the lower dispersion of the platinum metal. This is expected due to inherent disadvantages found in the production procedure and falls within normal literature values. The dependence of the performance on the dispersion value is displayed by the increased system performance at the same conditions (300 °C). A larger amount of material is necessary to achieve the desired performance as shown in the “minimum catalyst required” column, approximately 100% more. This increase in material use, when put into practice on a larger scale, will undoubtedly generate additional environmental burden.
Previously, in the product development analysis, results were presented comparing equal production amounts; however, this is an unfair comparison when less product is needed to achieve the same performance. The results below show the environmental burden from the production of these figures, 1 kg of catalyst from SCFD and 2.01 kg of catalyst from IWI.
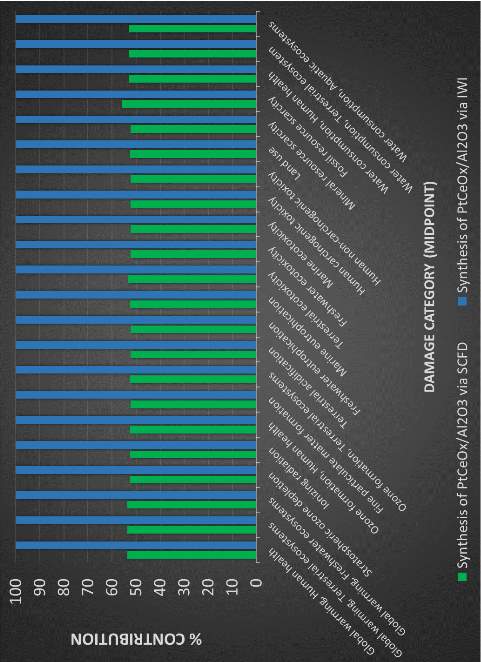
Figure 23: Impact of nanosystem performance on product development environmental burden (Midpoint)
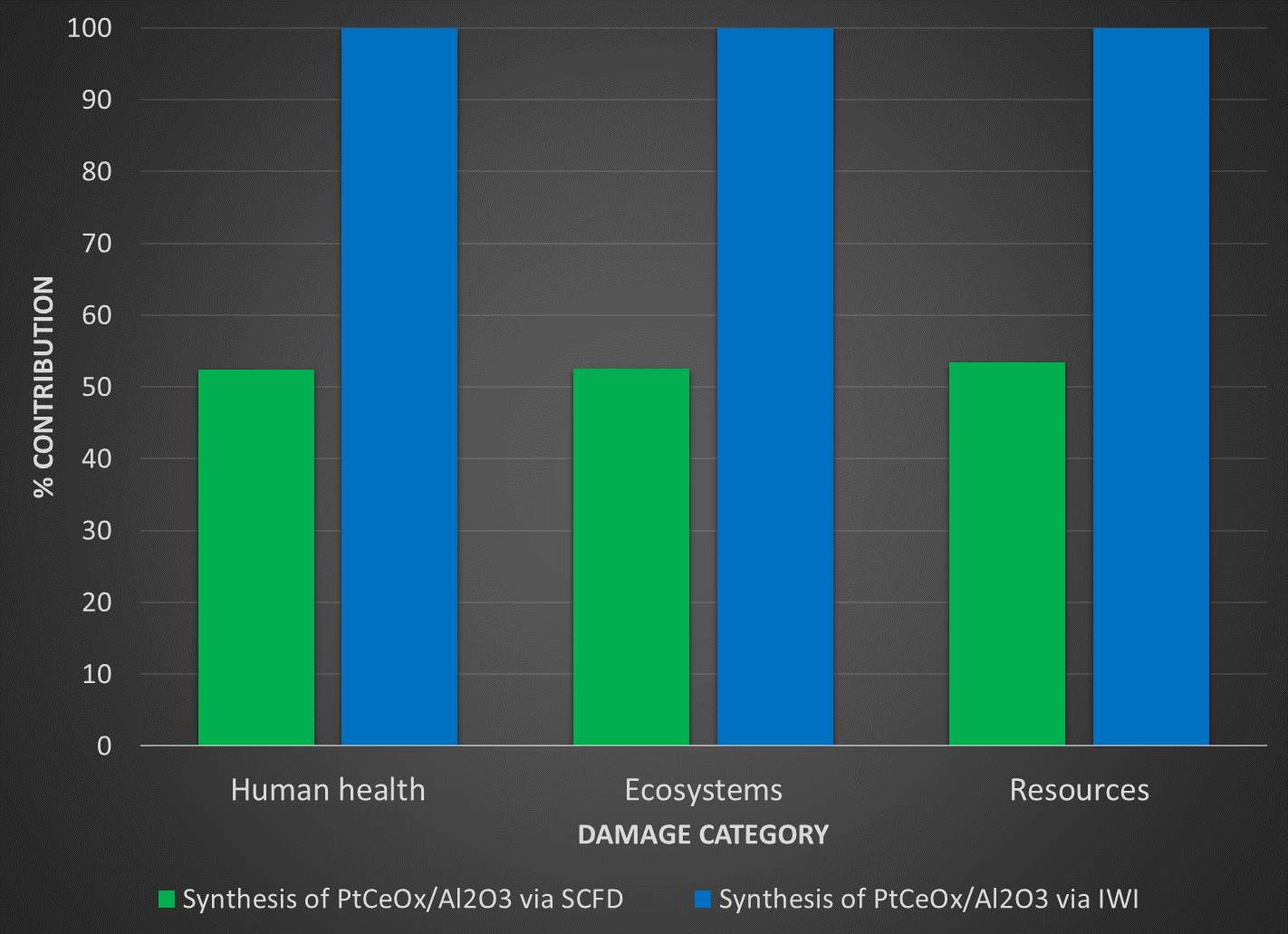
Figure 24: Impact of nanosystem performance on product development environmental burden (Endpoint)
We clearly see the impact of the increased performance observed in the catalyst prepared from a supercritical system. This can be mainly attributed to the reduction in platinum required to satisfy the CO requirement in the system of interest.
4.2.3.1 Highly Accelerated Life Testing
The initial design of experiment called for the subjection of the catalyst samples to higher than normal operating temperatures, essentially highly accelerated life testing (HALT). The melting point of platinum is 1768 °C and the equation for the minimum sintering temperature is129
Tmin = 0.3Tmelting
Therefore, sintering is expected to occur at temperatures above ~530 °C. For this study, the normal operating temperature is defined as 300 °C. The catalyst samples were subjected to temperatures from 600 °C to 1000 °C in 50 °C – 200 °C increments in an attempt to observe nucleation. These measurements were used to extrapolate a “lifetime” at 300 °C.
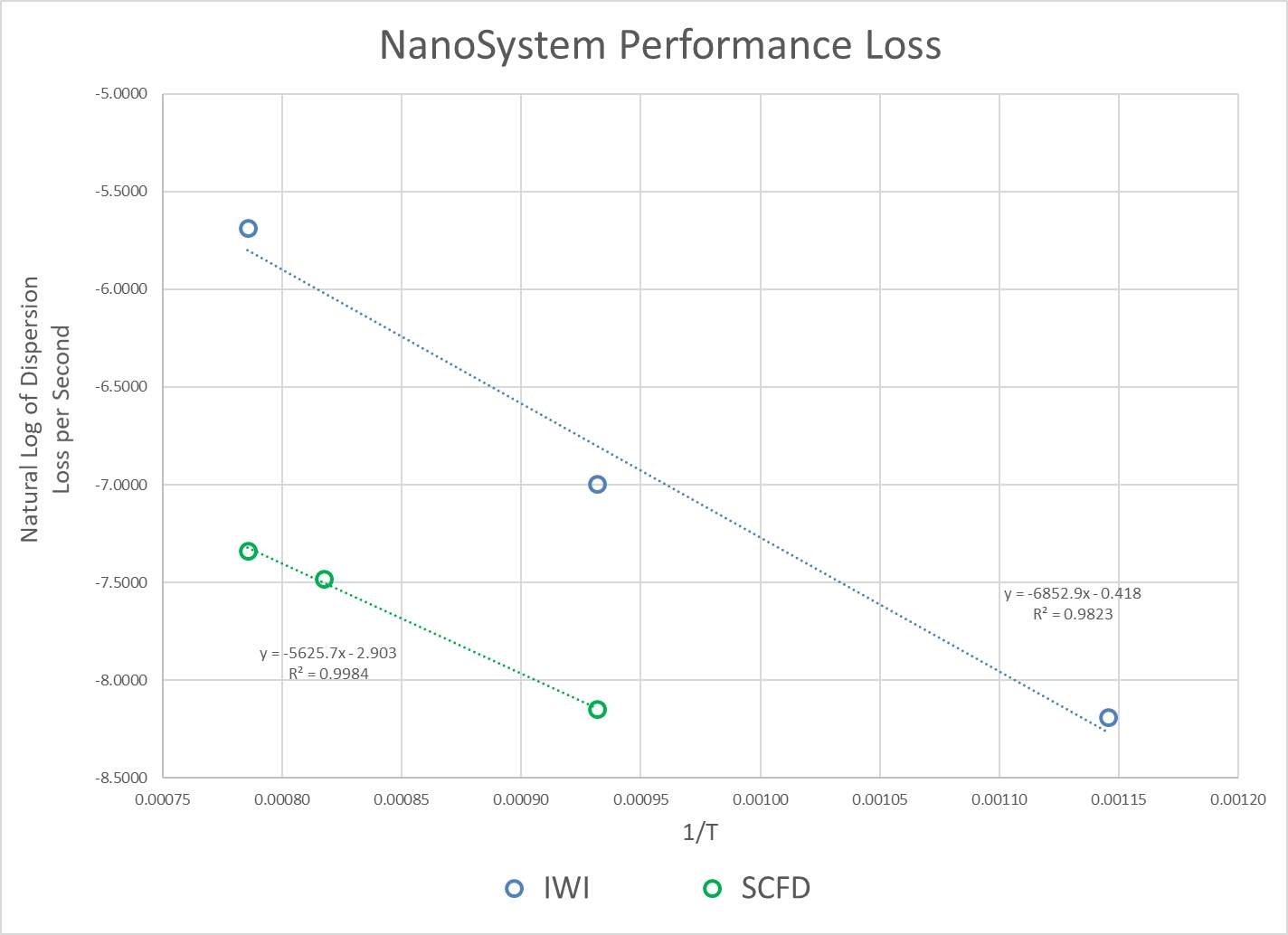
Figure 25: Highly accelerated life testing of nanosystems (graphical)
Table 7: Highly accelerated life testing of nanosystems (table)
| Nanosystem | Temperature (°C) | Dispersion of Pt (%) |
| IWI
Original dispersion = 20% |
600 | 19.0 |
| 800 | 16.7 | |
| 1000 | 7.8 | |
| SCFD
Original dispersion = 40% |
800 | 27.5 |
| 950 | 15.7 | |
| 1000 | 11.8 |
The variation in temperature range was due to differing resistance to agglomeration. The IWI catalyst showed loss at 600°C while the SCFD catalyst was resistant up to 800°C. The high dispersion of Pt on the SCFD catalyst results in a more robust system, inevitably leading to a longer lifetime. For life cycle assessment purposes, the catalyst was deemed “spent” once it had reach 10% dispersion. The rate of loss at 300°C produced an estimated life of 27.4 days and 38.6 days for the catalyst, a 34% difference. This translates into less material replacement, and subsequently, less overall production volume required. The LCA below shows the difference in the environmental burden when this is taken into account. The production amounts are 1000 kg of IWI catalyst and 709 kg of SCFD catalyst, calculated from the difference in estimated life between the two nanosystems.
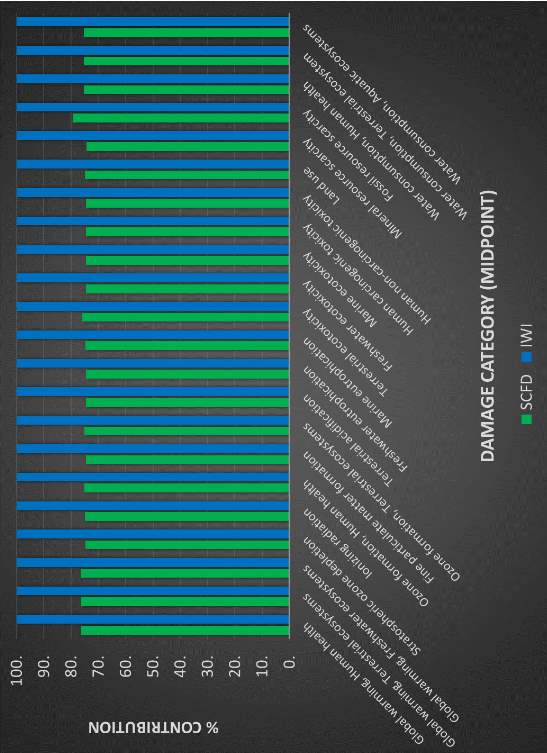
Figure 26: Influence of nanosystem robustness on production phase burden (midpoint)
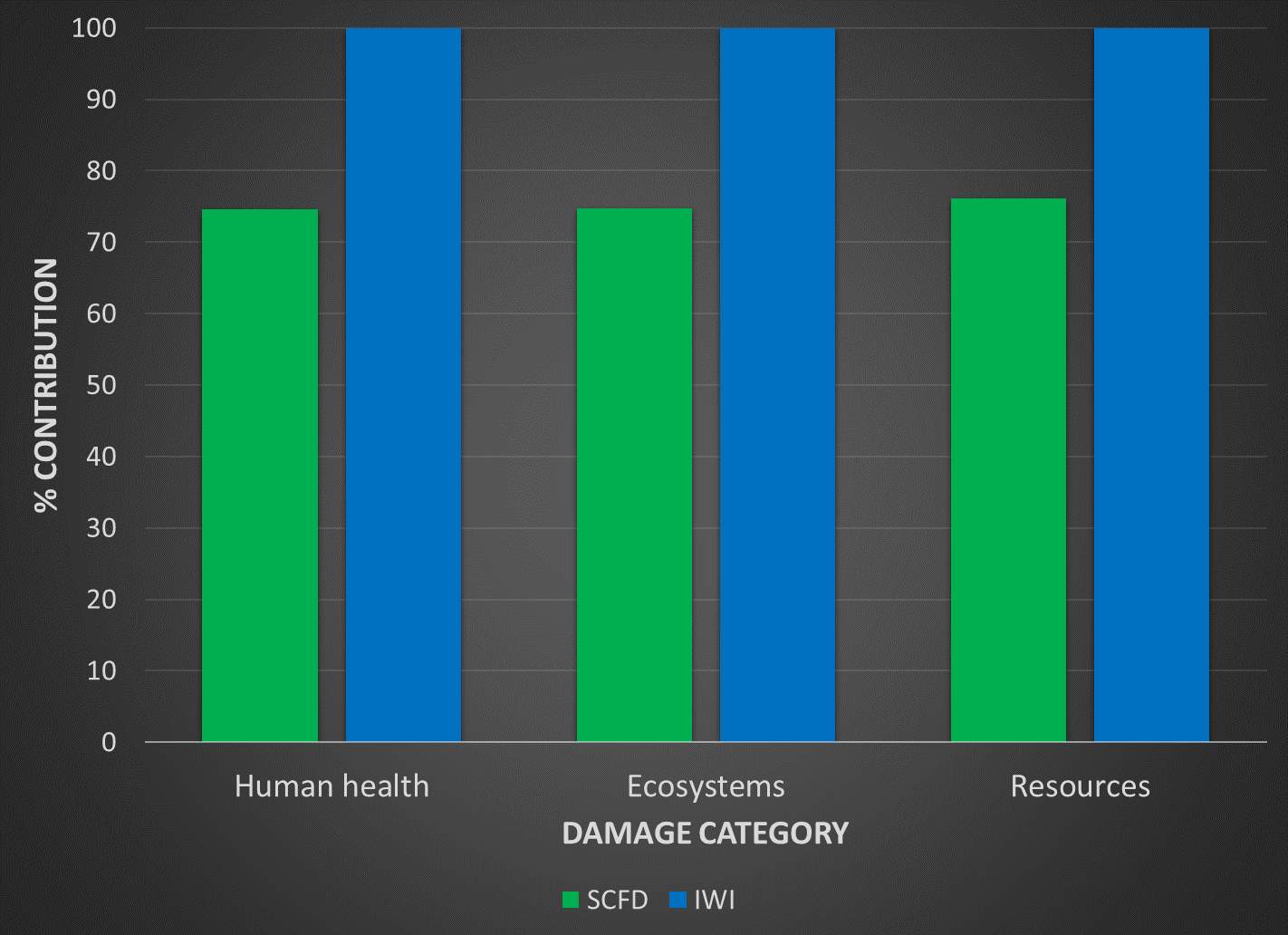
Figure 27: Influence of nanosystem robustness on production phase burden (endpoint)
These results only take into account the increased lifetime of the products in the production amount, not any additional benefits from increased performance. The resistance to degradation exhibited by the SCFD nanosystem reduces the environmental burden by approximately 25% across all three endpoint categories.
Further literature review notes that nucleation is not a typical concern in Pt/CeO2 fuel cell catalysts but rather carbonates blocking active Pt sites.130 As such, each catalyst was also ran at normal operation for 18 hours to check for a similar deactivation mechanism. Deactivation was not observed in either catalyst. This could be in part to the addition of alumina to the catalyst nanosystem and/or the large availability of active Pt sites which leads to a high turnover frequency/product performance.
The life testing demonstrates that there is a difference in the surface morphology between both nanosystems. The high dispersion influences the rate of degradation or sintering of platinum particles. This leads to a longer estimated life.
4.3 End of Life
The environmental burden of the platinum mining process and the associated economics necessitate that it be recycled. Fortunately, the composition of the catalyst is similar to that of a honeycomb type automotive catalytic converter and/or a reforming catalyst and current technologies can recover nearly 100% of the components.117,131
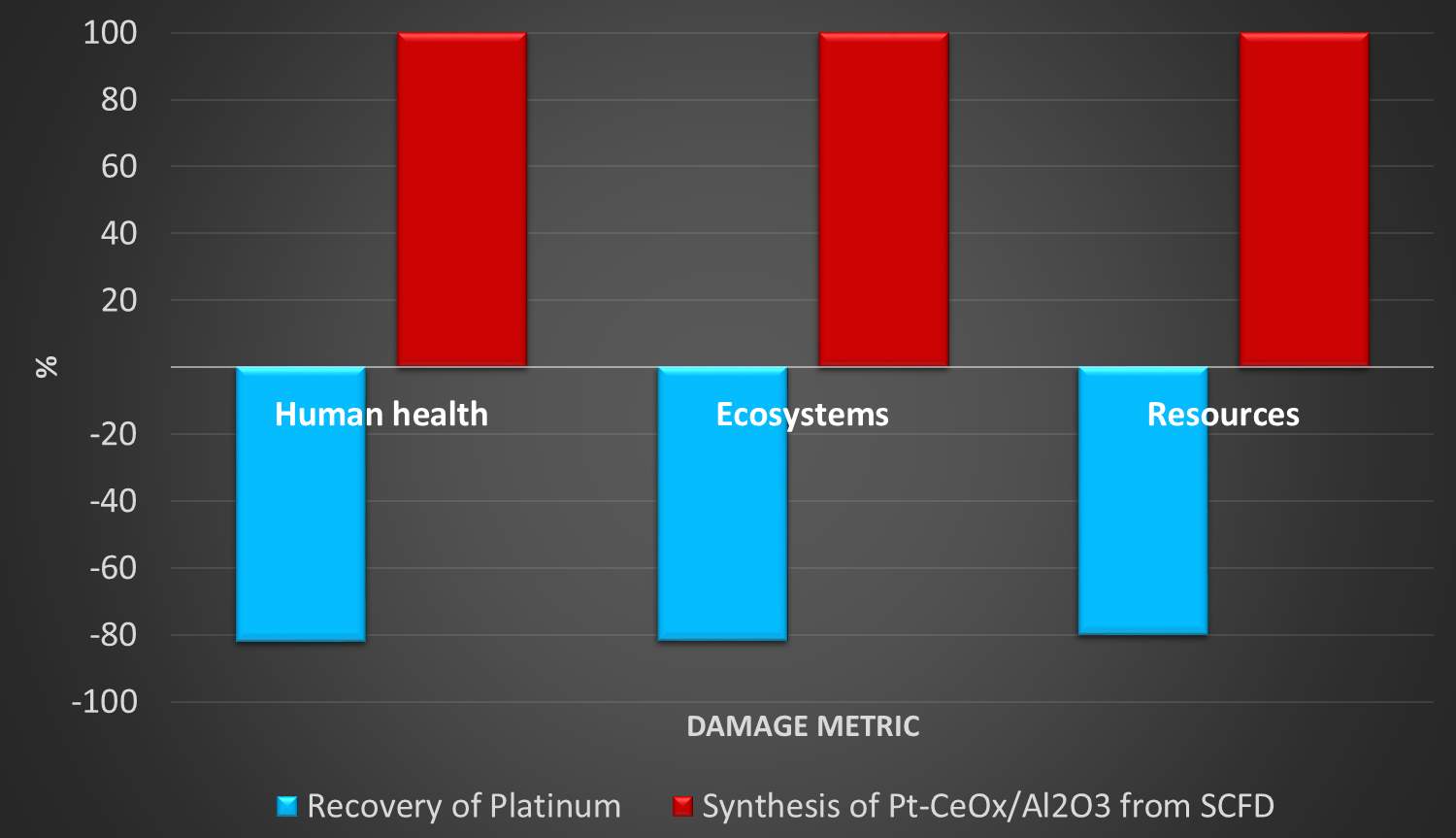
Figure 28: Effect of platinum recovery on product development
Figure 26 displays the change in environmental burden when recycling is applied at the SCFD catalysts’ end of life. The recycling process is based on the recovery of platinum group metals from spent automotive catalytic converters. The y-axis ranges from -100% to 100%. Negative values indicate mitigation of environmental damage. The separation and purification of platinum from the spent catalyst is much less intensive, both economically and environmentally, than virgin mining. Earlier results show that the incorporation of platinum results in 99.7% of the total burden in the product development phase. Although almost all the platinum is recovered, additional energy and materials in the recovery process contribute to the burden. Platinum loss also occurs from process inefficiencies. Therefore, recovery of 9.5 grams of platinum from the spent catalyst mitigates approximately 80% of the initial burden. This number could be improved upon, as the other components of the catalyst (ceria and alumina) are assumed as waste. In 2010, a look at the beverage industry showed that at then-current recycling rates, 250 MJ of energy was saved per 1000 aluminum cans.132 The recycling process results in an improvement in the overall impact of the catalyst’ life cycle for both pathways in spite of additional energy and materials needed to perform the recovery. As global consumption continues to increase, secondary platinum sources will become increasingly important.
CHAPTER V
VALIDATION & RESULTS
This chapter summarizes the analysis in Chapter IV and presents the Monte Carlo simulations performed for hypothesis validation. The sections are presented in a similar fashion as in Chapter IV. Validation was performed for each area analyzed in Chapter IV: product development phase, use phase, and finally the overall product life cycle. The main results from the simulations are shown in graphical form with accompanying data tables. The full results of the simulations can be found in the appendices.
5.1 Product Development Phase Monte Carlo Simulation
For validation of the product development phase comparison between the two product pathways, both production amounts were set at 1000 kg for ease of understanding. The Monte Carlo simulation was performed until the standard error of the mean (SEM) was 0.005 (99% confidence interval, =0.01) for the human health metric (DALY). The table and graph below displays the output from the simulation.
Table 8: Monte Carlo simulation results from product development phase
| Damage category | Ecosystems | Human health | Resources |
| SCFD ≥ IWI | 100% | 100% | 100% |
| Mean | 0.000364 | 0.313 | =2.64E+03 |
| Median | 0.00036 | 0.298 | 2.62E+03 |
| SD | 4.10E-05 | 0.0711 | 211 |
| CV | 11.30% | 22.70% | 8% |
| 0.50% | 0.000256 | 0.22 | 2.16E+03 |
| 99.50% | 0.000509 | 0.845 | 3.38E+03 |
| SEM | 2.88E-06 | 0.00499 | 14.8 |
| Runs | 203 |
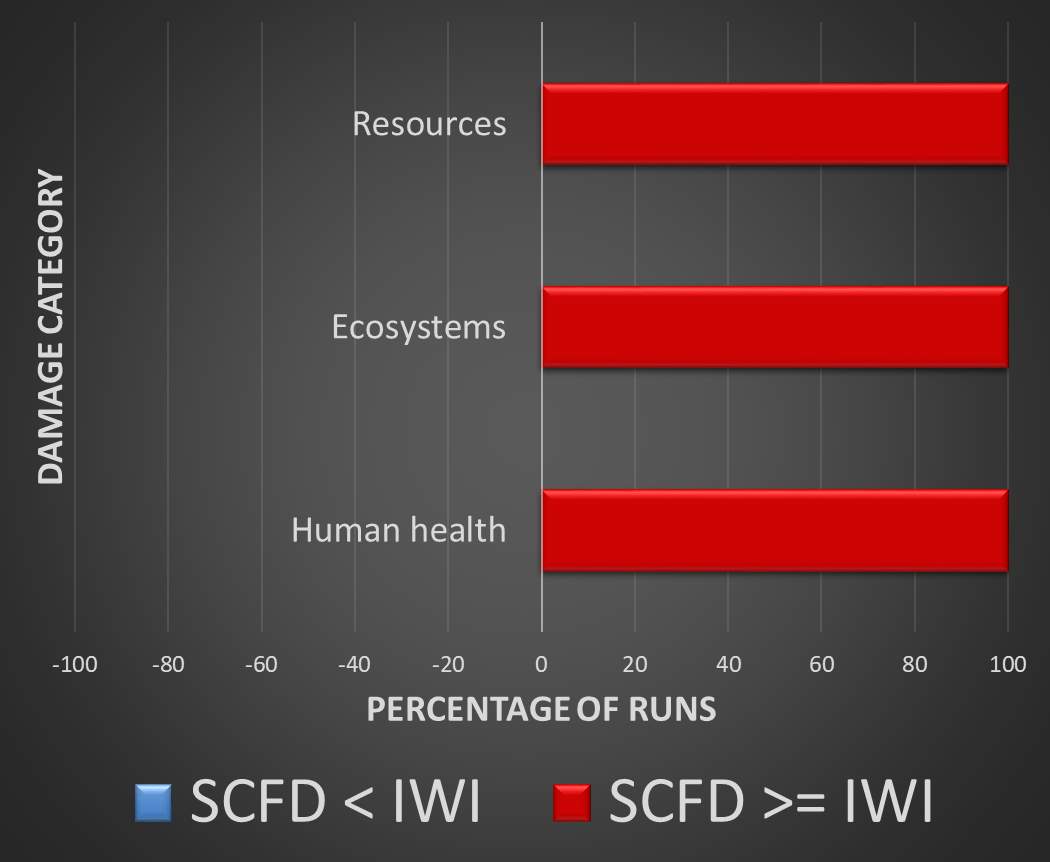
Figure 29: Monte Carlo simulation results for product development phase (graphical)
Of 203 runs, no scenario was found where the SCFD product pathway contributed less environmental burden than the IWI product pathway. This is attributed to two key areas in the SCFD product development path:
• The increased energy requirement to achieve supercritical conditions
• Additional energies, materials, and transport required to synthesize the SCFD platinum precursor
5.2 Use Phase Monte Carlo Simulation
For validation of the use comparison between the two product pathways, the production amount of SCFD product was set at 100 kg. Taking into account the performance ratio calculated earlier in Chapter IV, the production amount of IWI product was set at 201 kg. The Monte Carlo simulation was performed until the standard error of the mean (SEM) was 0.005 (99% confidence interval, =0.01) for the human health metric (DALY). The table and graph below displays the output from the simulation.
Table 9: Monte Carlo simulation results from product development phase with use phase contribution
| Damage category | Ecosystems | Human health | Resources |
| SCFD ≥ IWI | 0% | 0% | 0% |
| Mean | -0.000655 | -0.585 | -3.32E+03 |
| Median | -0.00065 | -0.56 | -3.31E+03 |
| SD | 8.18E-05 | 0.134 | 355 |
| CV | -12.50% | -22.90% | -10.70% |
| 0.50% | -0.00109 | -1.37 | -4.47E+03 |
| 99.50% | -0.000477 | -0.395 | -2.53E+03 |
| SEM | 3.04E-06 | 0.005 | 13.2 |
| Runs | 722 |
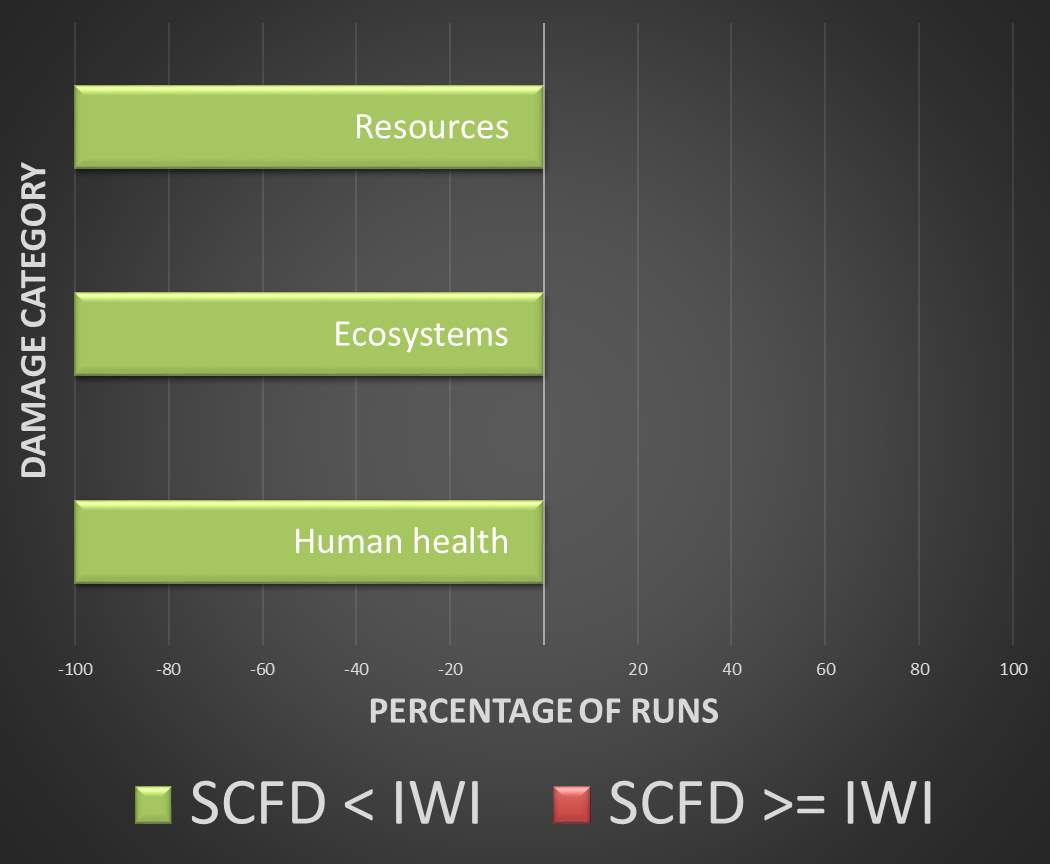
Figure 30: Monte Carlo simulation results for product development with use phase contribution (graphical)
Out of 722 runs, no scenario was found where the SCFD product pathway contributed more environmental burden than the IWI product pathway when the enhanced performance in the use phase was taken into account. This is attributed to less platinum mining.
5.3 Overall Product Life Cycle Monte Carlo Simulation
For validation of the overall product life cycles and their respective comparison, the production amount of SCFD product was set at 100 kg. Taking into account the performance ratio calculated earlier in Chapter IV, the production amount of IWI product was set at 201 kg. The Monte Carlo simulation was performed until the standard error of the mean (SEM) was 0.005 (99% confidence interval, =0.01) for the human health metric (DALY). The table and graph below displays the output from the simulation.
Table 10: Monte Carlo simulation results from overall product life cycle with use phase performance contribution
| Damage category | Ecosystems | Human health | Resources |
| SCFD ≥ IWI | 0% | 0% | 0% |
| Mean | -0.00036 | -0.318 | -1.82E+03 |
| Median | -0.00035 | -0.302 | -1.80E+03 |
| SD | 6.92E-05 | 0.0752 | 192 |
| CV | -19.30% | -23.70% | -10.50% |
| 0.50% | -0.00109 | -0.934 | -2.41E+03 |
| 99.50% | -0.00025 | -0.218 | -1.39E+03 |
| SEM | 4.58E-06 | 0.00498 | 12.7 |
| Runs | 228 |
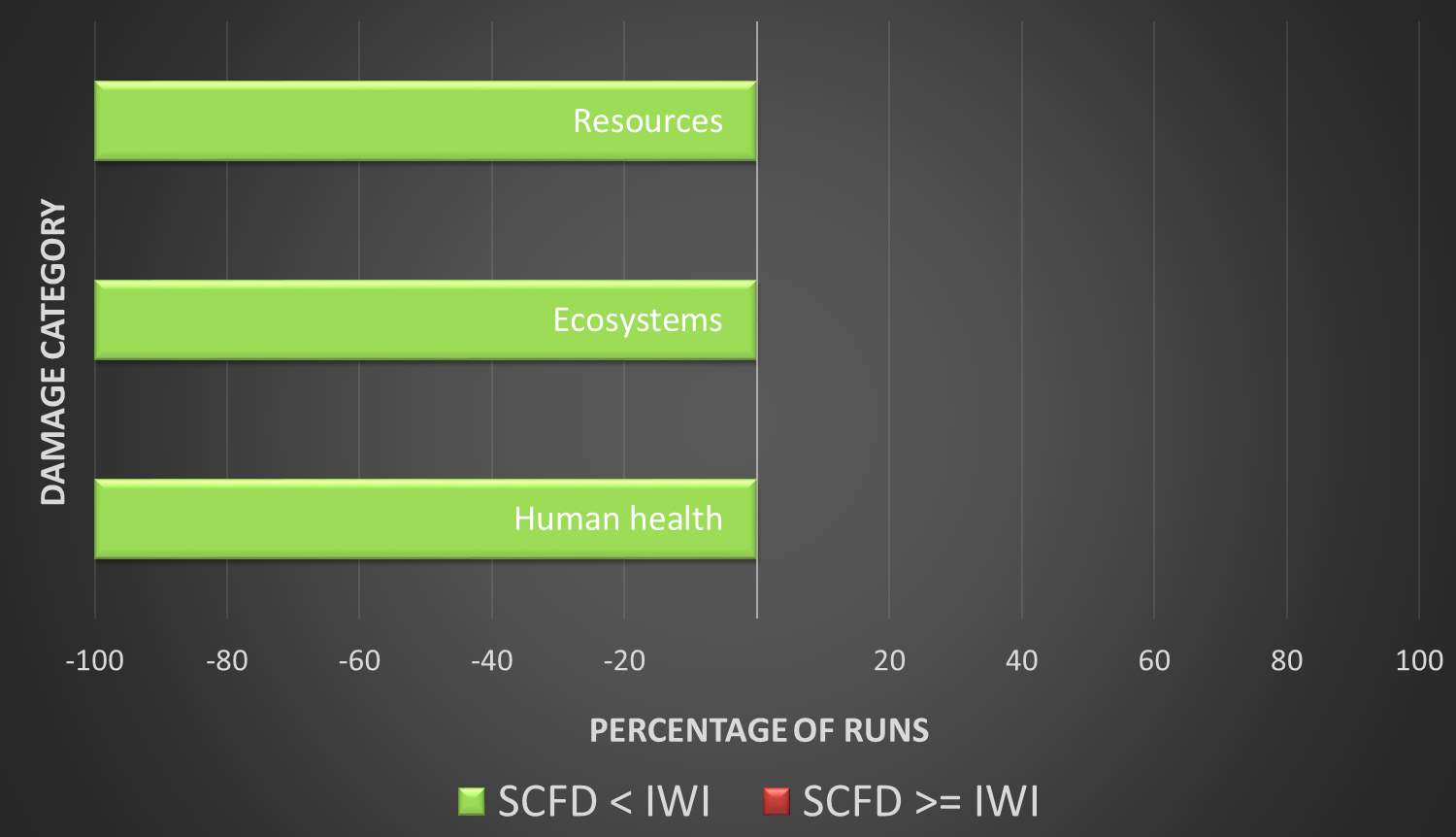
Figure 31: Monte Carlo simulation of overall product life cycles with performance contribution
No scenario was found in 228 runs in which overall life cycle of the SCFD produced catalyst exhibited a higher environmental burden. This holds true for all three damage metrics: damage to human health, damage to ecosystems, and resource depletion. The Monte Carlo simulation performed for the overall life cycle validates the hypothesis of this study:
Alumina-supported platinum/ceria catalysts for water-gas shift reaction prepared from a supercritical fluid deposition system contribute less potential environmental damage than those prepared by incipient wetness impregnation
CHAPTER VI
CONCLUSIONS
6.1 Summary
Inherent characteristics of supercritical fluid deposition mitigate the increased energy consumption upstream required for the supercritical process’s operating conditions. Not only does the method result in increased efficiency and optimized precious metal use, the anchoring of the particles on the substrate’s surface contribute much needed longevity to the final product itself. An increased life span impacts several areas: overall system life, less waste from recycling processes, and less environmental damage from mining operations. This research is an example of the importance of taking a holistic approach to chemical process and material design, especially processes that make use of precious metals. It serves as a general framework for the life cycle assessment of nanosystems and stresses the importance of material selection when assessing environmental burden.
The main limitation of this study lay in the assumptions made for both the commercial scale supercritical process and the data used to build the life cycle inventory. Supercritical fluid deposition of metals from supercritical CO2 is still a new area of research interest in the catalyst community. As such, there are several “unknowns” that could lead to process optimization and a reduced environmental footprint. According to Sauser and Ramirez-Marquez, there are two metrics needed to determine a system’s readiness level (SRL): integration readiness level (IRL) and technology readiness level (TRL).133
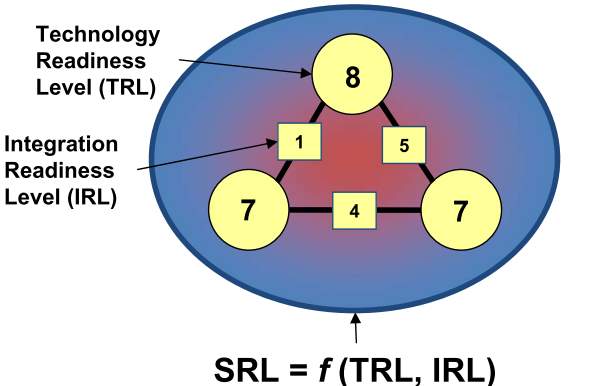
Figure 32: System readiness level and relationships133
With supercritical processes already implemented industrially, integration should not be a challenge. Difficulties arise in the technology itself. Specifically, the nature of the interface between the dissolved platinum precursor and the alumina support. Understanding this interface will lead to optimal process conditions, a further decrease in energy use, and add to the system readiness level. This will increase the viability of industrial production of precious metal-based catalysts, particularly during the conduction of alternative comparisons and trade studies.
6.2 Description of Future Research
Life cycle assessment inherently has a large degree of uncertainty associated with it. This is without the additional uncertainty introduced from estimates for energy, transportation, water consumption, emissions and reaction efficiency data. The accuracy of these values can be refined through additional literature review, consultation with industry experts, and additional laboratory experiment. An interesting parameter to be considered is the year of the study. From this study, the source of energy in nearly all processes are carbonaceous fuels. This could be in part to the current energy mixture in a region or limitations on the machinery being used. From the 2016 World Energy Outlook, it is projected that by 2040, renewable energies will be competitive without subsidies.134 These projections could influence the conclusions from the study. Scenarios using projected energy mixtures could be studied to ascertain the influence of electricity sources on the life cycle assessment.
This study uses the hierarchist exclusively. Results may differ when another perspective is used. Additional validation could be sought through implementing the individualist and egalitarian perspectives.
The influence of energy mixtures is just one example. Systems engineering, by definition, is an interdisciplinary field. From this research, there are many areas that could be explored in future work. The following areas are of particular relevance to this work:
- Investigation of “real world” fuel cell gas compositions to assess catalyst performance, both in reaction rate and resistance to deactivation
- Optimization of the supercritical fluid deposition system through variation in
- Operating parameters
- Precursor selection
- Economic analysis of product life cycle for SCFD and IWI prepared products and the influence of an extended life of operation
- Continuous deposition of metals from supercritical CO2 vs. batch deposition and the recovery of energy from a supercritical process
- Life cycle assessment for WGSR catalysts found in literature normalized according to their respective performance rates
- Substitution of platinum metal with other precious and/or non-precious metals and their environmental burden
REFERENCES
REFERENCES
1. Anastas, P. T. & Williamson, T. C. Green Chemistry : An Overview. (1996).
2. Anastas, P. T. & Zimmerman, J. B. Design through the 12 principles of green engineering. IEEE Eng. Manag. Rev. 35, 16 (2007).
3. Rebitzer, G. et al. Life Cycle Assessment: Framework, goal and scope definition, inventory analysis, and applications. Environ. Int. 30, 701–720 (2004).
4. NASA. NASA Systems Engineering Handbook. Syst. Eng. 6105, 287 (2016).
5. Todd, J. A. & Curran, M. A. Streamlined Life-Cycle Assessment: A Final Report from the SETAC North America Streamlined LCA Workgroup. Environ. Toxicol. 31 (1999).
6. Hofstetter, P. Perspectives in life cycle impact assessment: A Structured Approach to Combine Models of the Technospere, Ecosphere, and Valuesphere. (Swiss Federal Institute of Technology Zurich, 1998). doi:10.3929/ethz-a-010782581
7. Hansen, T. W., Delariva, A. T., Challa, S. R. & Datye, A. K. Sintering of catalytic nanoparticles: Particle migration or ostwald ripening? Acc. Chem. Res. 46, 1720–1730 (2013).
8. Charpentier, J. C. The triplet ‘molecular processes-product-process’ engineering: The future of chemical engineering? Chem. Eng. Sci. 57, 4667–4690 (2002).
9. Leitner, W. Supercritical carbon dioxide as a green reaction medium for catalysis. Acc. Chem. Res. 35, 746–756 (2002).
10. Poliakoff, M. et al. Supercritical fluids: Clean solvents for green chemistry. Chinese J. Chem. 17, 212–222 (1999).
11. Sheldon, R. A. Green solvents for sustainable organic synthesis: state of the art. Green Chem. 7, 267 (2005).
12. DeSimone, J. M. Practical Approaches to Green Solvents. Science (80-. ). 297, 799–803 (2002).
13. Rodríguez-Meizoso, I. et al. Life cycle assessment of green pilot-scale extraction processes to obtain potent antioxidants from rosemary leaves. J. Supercrit. Fluids 72, 205–212 (2012).
14. 2008, I. ISO/IEC 15288: Systems and software engineering — System life cycle processes. (2008).
15. Charpentier, J. C. & McKenna, T. F. Managing complex systems: Some trends for the future of chemical and process engineering. Chem. Eng. Sci. 59, 1617–1640 (2004).
16. IPCC. Summary for Policymakers. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (2014). doi:10.1017/CBO9781107415324
17. Anastas, P. T.; Warner, J. C. Green Chemistry: Theory and Practice. (Oxford University Press, 1998). doi:10.1159/000143289
18. Corma, A. & Garcia, H. Silica-bound homogenous catalysts as recoverable and reusable catalysts in organic synthesis. Adv. Synth. Catal. 348, 1391–1412 (2006).
19. Azapagic, A. & Clift, R. The application of life cycle assessment to process optimisation. Comput. Chem. Eng. 23, 1509–1526 (1999).
20. Scott, J. H. Fuel Cell Development for NASA ’ s Human Exploration Program : Benchmarking With “ The Hydrogen Economy ”. (2016). doi:10.1115/1.2972170
21. Koroneos, C., Dompros, A., Roumbas, G. & Moussiopoulos, N. Life cycle assessment of hydrogen fuel production processes. 29, 1443–1450 (2004).
22. Griffiths, O. G. et al. Identifying the largest environmental life cycle impacts during carbon nanotube synthesis via chemical vapour deposition. J. Clean. Prod. 42, 180–189 (2013).
23. Huntzinger, D. N. & Eatmon, T. D. A life-cycle assessment of Portland cement manufacturing: comparing the traditional process with alternative technologies. J. Clean. Prod. 17, 668–675 (2009).
24. Bhandari, R., Trudewind, C. A. & Zapp, P. Life cycle assessment of hydrogen production via electrolysis – A review. J. Clean. Prod. 85, 151–163 (2014).
25. Peng, J., Lu, L. & Yang, H. Review on life cycle assessment of energy payback and greenhouse gas emission of solar photovoltaic systems. Renew. Sustain. Energy Rev. 19, 255–274 (2013).
26. Tabone, M. D., Cregg, J. J., Beckman, E. J. & Landis, A. E. Sustainability Metrics: Life Cycle Assessment and Green Design in Polymers. Environ. Sci. Technol. 44, 826–8269 (2010).
27. Dufour, J., Serrano, D. P., Gálvez, J. L., Moreno, J. & García, C. Life cycle assessment of processes for hydrogen production. Environmental feasibility and reduction of greenhouse gases emissions. Int. J. Hydrogen Energy 34, 1370–1376 (2009).
28. Cherubini, F. & Ulgiati, S. Crop residues as raw materials for biorefinery systems – A LCA case study. Appl. Energy 87, 47–57 (2010).
29. Stark, A. et al. Microwave-assisted Kolbe-Schmitt synthesis using ionic liquids or dimcarb as reactive solvents. Chem. Eng. Technol. 32, 1730–1738 (2009).
30. Kralisch, D. et al. Rules and benefits of Life Cycle Assessment in green chemical process and synthesis design: a tutorial review. J. Clean. Prod. 17, 123–145 (2015).
31. Fthenakis, V. M. & Kim, H. C. Photovoltaics: Life-cycle analyses. Sol. Energy 85, 1609–1628 (2011).
32. Suh, S. et al. System Boundary Selection in Life-Cycle Inventories Using Hybrid Approaches. Environ. Sci. Technol. 38, 1509–1526 (2010).
33. Jiménez-Gonzalez, C., Curzons, A. D., Constable, D. J. C. & Cunningham, V. L. Expanding GSK’s Solvent Selection Guide – Application of life cycle assessment to enhance solvent selections. Clean Technol. Environ. Policy 7, 42–50 (2004).
34. Herrmann, I. T. & Moltesen, A. Does it matter which Life Cycle Assessment (LCA) tool you choose? – A comparative assessment of SimaPro and GaBi. J. Clean. Prod. 86, 163–169 (2015).
35. Raluy, R. G., Serra, L., Uche, J. & Valero, A. Life-cycle assessment of desalination technologies integrated with energy production systems. Desalination 167, 445–458 (2004).
36. Sydnor, C., Marshall, T. & Mcginnis, S. Operational Phase Life Cycle Assessment of Select NASA Ground Test Facilities.
37. Khoo, H. H. & Tan, R. B. H. Life Cycle Investigation of CO 2 Recovery and Sequestration. Environ. Sci. Technol. 40, 4016–4024 (2006).
38. Salve, J. Dealing with uncertainties in Life Cycle Assessment for emerging technologies. Environmental Sciences (Open University of the Netherlands, 2008).
39. Tsang, M., Philippot, G., Aymonier, C. & Sonnemann, G. Anticipatory life-cycle assessment of supercritical fl uid synthesis of barium strontium titanate. 4924–4933 (2016). doi:10.1039/c6gc00646a
40. Ingwersen, W. et al. Detailed life cycle assessment of Bounty ® paper towel operations in the United States. J. Clean. Prod. 131, 509–522 (2016).
41. Frischknecht, R. et al. Overview and Methodology. ecoinvent Cent. 1–77 (2007).
42. Baayen, H. Eco-indicator 99 Manual for Designers. (2000).
43. Goedkoop, M. The Eco-indicator 99 A damage oriented method for Life Cycle Impact Assessment Methodology Annex. (2001).
44. Goedkoop, M. & Huijbregts, M. ReCiPe 2008. (2013).
45. Huijbregts, M. a. J. et al. ReCiPe 2016: A harmonized life cycle impact assessment method at midpoint and enpoint level – Report 1 : characterization. 194 (2016).
46. Goedkoop, M. & Spriensma, R. The Eco-indicator 99 A damage oriented method for Life Cycle Impact Assessment. (2001).
47. Ayres, R. U. Life cycle analysis: A critique. Resour. Conserv. Recycl. 14, 199–223 (1995).
48. Reap, J., Roman, F., Duncan, S. & Bras, B. A survey of unresolved problems in life cycle assessment. Part 2: Impact assessment and interpretation. Int. J. Life Cycle Assess. 13, 374–388 (2008).
49. The International Standards Organisation. Environmental management — Life cycle assessment — Principles and framework. Iso 14040 2006, 1–28 (2006).
50. LaVan, D. a, McGuire, T. & Langer, R. Small-scale systems for in vivo drug delivery. Nat. Biotechnol. 21, 1184–1191 (2003).
51. Vasir, J. K., Reddy, M. K. & Labhasetwar, V. D. Nanosystems in Drug Targeting: Opportunities and Challenges. Curr. Nanosci. 1, 47–64 (2005).
52. Tian, B. et al. Coaxial silicon nanowires as solar cells and nanoelectronic power sources. Nature 449, 885–889 (2007).
53. Davis, M. E., Katz, A. & Ahmad, W. R. Rational Catalyst Design via Imprinted Nanostructured Materials. Chem. Mater. 8, 1820–1839 (1996).
54. Ariga, K., Mori, T. & Hill, J. P. Mechanical control of nanomaterials and nanosystems. Adv. Mater. 24, 158–176 (2012).
55. Rashkeev, S. N., Dai, S. & Overbury, S. H. Modification of Au/TiO2 nanosystems by SiO2 monolayers: Toward the control of the catalyst activity and stability. J. Phys. Chem. C 114, 2996–3002 (2010).
56. Bukhtiyarov, V. I. & Slin’ko, M. G. Metallic nanosystems in catalysis. Russ. Chem. Rev. 70, 147–159 (2007).
57. Barreca, D. et al. The potential of supported Cu2O and CuO nanosystems in photocatalytic H2 production. ChemSusChem 2, 230–233 (2009).
58. Sample, J. & Charles Jr., H. Systems Engineering at the Nanoscale. John Hopkins APL Tech. Dig. 31, 50–57 (2012).
59. Jacobs, G., Patterson, P. M., Graham, U. M., Crawford, A. C. & Davis, B. H. Low temperature water gas shift: The link between the catalysis of WGS and formic acid decomposition over Pt/ceria. Int. J. Hydrogen Energy 30, 1265–1276 (2005).
60. Lloyd, S. M., Lave, L. B. & Matthews, H. S. Life cycle benefits of using nanotechnology to stabilize platinum-group metal particles in automotive catalysts. Environ. Sci. Technol. 39, 1384–1392 (2005).
61. Holman, P. A., Shonnard, D. R. & Holles, J. H. Using Life Cycle Assessment to Guide Catalysis Research. Ind. Eng. Chem. Res. 48, 6668–6674 (2009).
62. Milani, A. S., Eskicioglu, C., Robles, K., Bujun, K. & Hosseini-Nasab, H. Multiple criteria decision making with life cycle assessment for material selection of composites. Express Polym. Lett. 5, 1062–1074 (2011).
63. Walser, T., Demou, E., Lang, D. J. & Hellweg, S. Prospective Environmental Life Cycle Assessment of Nanosilver. Environ. Sci. Technol. 45, 4570–4578 (2011).
64. Marquis, B. J., Love, S. A., Braun, K. L. & Haynes, C. L. Analytical methods to assess nanoparticle toxicity. Analyst 134, 425 (2009).
65. Mueller, N. C. & Nowack, B. Exposure modelling of engineered nanoparticles in the environment. Environ. Sci. Technol. 42, 44447–53 (2008).
66. Ann, M. & Irving, S. Nanotechnology and Life Cycle Assessment . A systems approach to Nanotechnology and the environment Synthesis of Results Obtained at a Workshop Washington , DC 2 – 3 October 2006. (2007).
67. Baiker, A. Supercritical Fluids in Heterogeneous Catalysis. Chem. Rev. 99, 453–474 (1999).
68. Garrido, G. I. et al. Supercritical deposition of Pt on SnO2-coated Al2O3 foams: Phase behaviour and catalytic performance. Appl. Catal. A Gen. 338, 58–65 (2008).
69. Zhang, Y. & Erkey, C. Preparation of supported metallic nanoparticles using supercritical fluids: A review. J. Supercrit. Fluids 38, 252–267 (2006).
70. Herrero, M., Mendiola, J. A., Cifuentes, A. & Ibáñez, E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A 1217, 2495–2511 (2010).
71. Brunner, G. Applications of Supercritical Fluids. Annu. Rev. Chem. Biomol. Eng. 1, 321–342 (2010).
72. Brunner, G. Supercritical fluids: Technology and application to food processing. J. Food Eng. 67, 21–33 (2005).
73. Peker, H., Srinivasan, M. P., Smith, J. M. & McCoy, B. J. Caffeine extraction rates from coffee beans with supercritical carbon dioxide. AIChE J. 38, 761–770 (1992).
74. Lack, E. & Seidlitz, H. in Extraction of Natural Products Using Near-Critical Solvents (eds. King, M. B. & Bott, T. R.) 101–139 (Springer Netherlands, 1993). doi:10.1007/978-94-011-2138-5_5
75. Lack, E. & Seidlitz, H. Commercial Scale Decaffeination of Coffee and Tea Using Supercritical CO2.
76. Attard, T. M., McElroy, C. R. & Hunt, A. J. Economic assessment of supercritical CO<inf>2</inf> extraction of waxes as part of a maize stover biorefinery. Int. J. Mol. Sci. 16, 17546–17564 (2015).
77. Pehnt, M. Dynamic life cycle assessment (LCA) of renewable energy technologies. Renew. Energy 31, 55–71 (2006).
78. Gunay, M. E., Akpinar, F., Onsan, Z. I. & Yildirim, R. Investigation of water gas-shift activity of Pt-MOx-CeO2/Al2O3 (M = K, Ni, Co) using modular artificial neural networks. Int. J. Hydrogen Energy 37, 2094–2102 (2012).
79. Gosavi, P. V. & Biniwale, R. B. Catalytic preferential oxidation of carbon monoxide over platinum supported on lanthanum ferriteeceria catalysts for cleaning of hydrogen. J. Power Sources 222, 1–9 (2013).
80. Melorose, J., Perroy, R. & Careas, S. Spillover and Mobility of Species on Solid Surfaces. Studes in Surface Science and Catalysis 138, (2001).
81. Spieker, W. A. & Regalbuto, J. R. A fundamental model of platinum impregnation onto alumina. Chem. Eng. Sci. 56, 3491–3504 (2001).
82. Bagheri, S., Muhd Julkapli, N. & Bee Abd Hamid, S. Titanium dioxide as a catalyst support in heterogeneous catalysis. ScientificWorldJournal 2014, 727496 (2014).
83. Schwarz, J. A., Contescu, C. & Contescu, A. Methods for Preparation of Catalytic Materials. (1995).
84. Li, J. et al. New method for the preparation of nonuniform distributed Co/SiO2 catalysts. Chem. Commun. (Camb). 5954–6 (2008). doi:10.1039/b813641f
85. Xu, C. et al. Low temperature CO oxidation over unsupported nanoporous gold. J. Am. Chem. Soc. 129, 42–43 (2007).
86. Oudenhuijzen, M. K., Kooyman, P. J., Tappel, B., van Bokhoven, J. A. & Koningsberger, D. C. Understanding the Influence of the Pretreatment Procedure on Platinum Particle Size and Particle-Size Distribution for SiO2 Impregnated with [Pt2+(NH3)4](NO3−)2: A Combination of HRTEM, Mass Spectrometry, and Quick EXAFS. J. Catal. 205, 135–146 (2002).
87. Wolff, S. et al. The Journal of Supercritical Fluids Preparation of supported Pt nanoparticles by supercritical fluid reactive deposition : Influence of precursor , substrate and pressure on product properties. J. Supercrit. Fluids 95, 588–596 (2014).
88. Lin, Y. & Cui, X. Pt and Pt-Ru/Carbon Nanotube Nanocomposites Synthesized in Supercritical Fluid as Fuel Cell Electrocatalysts. Proc. – Electrochem. Soc. 2005–14, 255–261 (2008).
89. Erkey, C. Preparation of metallic supported nanoparticles and films using supercritical fluid deposition. J. Supercrit. Fluids 47, 517–522 (2009).
90. Hui, W., Mammucari, R. & Foster, N. R. Solubility of organometallic complexes in supercritical carbon dioxide : A review. J. Organomet. Chem. 724, 102–116 (2013).
91. Caban, A., Long, D. P. & Watkins, J. J. Deposition of Gold Films and Nanostructures from Supercritical Carbon Dioxide. Society 2028–2033 (2004).
92. Deal, J. W., Le, P., Corey, C. B., More, K. & Wheeler, C. The Journal of Supercritical Fluids Water-gas shift reaction on alumina-supported Pt-CeO x catalysts prepared by supercritical fluid deposition. J. Supercrit. Fluids 119, 113–121 (2017).
93. Zhang, Y., Kang, D., Saquing, C., Aindow, M. & Erkey, C. Supported platinum nanoparticles by supercritical deposition. Ind. Eng. Chem. Res. 44, 4161–4164 (2005).
94. Germani, G., Schurrman, Y. Water-Gas Shift Reaction Kinetics Over ? -Structured Pt/CeO2/Al2O3 Catalysts. Am. Inst. Chem. Eng. 52, 1806–1813 (2006).
95. Kaya, B., Irmak, S., Hesenov, A., Erbatur, O. & Erkey, C. Effect of support materials on supported platinum catalyst prepared using a supercritical fluid deposition technique and their catalytic performance for hydrogen-rich gas production from lignocellulosic biomass. Bioresour. Technol. 123, 723–726 (2012).
96. D.Shekhawat, Spivey, J. J. & Berry, D. a. Processing, Fuel Cells: Technologies for fuel processing. (2011).
97. Gawande, M. B., Pandey, R. K. & Jayaram, R. V. Role of mixed metal oxides in catalysis science—versatile applications in organic synthesis. Catal. Sci. Technol. 2, 1113 (2012).
98. Ratnasamy, C., Wagner, J. P., Ratnasamy, C. & Wagner, J. P. Water Gas Shift Catalysis Water Gas Shift Catalysis. Catal. Rev. – Sci. Eng. 51, 325–440 (2009).
99. Knez et al. Industrial applications of supercritical fluids: A review. Energy 77, 235–243 (2014).
100. Germani, G., Alphonse, P., Courty, M., Schuurman, Y. & Mirodatos, C. Platinum/ceria/alumina catalysts on microstructures for carbon monoxide conversion. Catal. Today 110, 114–120 (2005).
101. Heede, P. Van Den & Belie, N. De. Cement & Concrete Composites Environmental impact and life cycle assessment ( LCA ) of traditional and ‘ green ’ concretes : Literature review and theoretical calculations. Cem. Concr. Compos. 34, 431–442 (2012).
102. Lundin, M., Bengtsson, M. & Molander, S. Life cycle assessment of wastewater systems: Influence of system boundaries and scale on calculated environmental loads. Environ. Sci. Technol. 34, 180–186 (2000).
103. Carbon Dioxide. Ullmann’s Encyclopedia of Industrial Chemistry 7th Edition 8–11 (2005).
104. EPA. Life Cycle Assessment: Principles and Practice. Vasa 88 (2008). doi:10.1016/j.marpolbul.2007.03.022
105. Lemmon, E. W. W., Huber, M. L. L. & McLinden, M. O. O. NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties (REFPROP), Version 9.0. Phys. Chem. Prop. … (2010). doi:10.1234/12345678
106. Bozbag, S. E. et al. Thermodynamic control of metal loading and composition of carbon aerogel supported Pt-Cu alloy nanoparticles by supercritical deposition. J. Phys. Chem. C 117, 6777–6787 (2013).
107. Manasilp, A. & Gulari, E. Selective CO oxidation over Pt / alumina catalysts for fuel cell applications. 37, 17–25 (2002).
108. Phatak, A. A. et al. Kinetics of the water-gas shift reaction on Pt catalysts supported on alumina and ceria. Catal. Today 123, 224–234 (2007).
109. Souza, M. M. V. M., Aranda, D. A. G. & Schmal, M. Reforming of Methane with Carbon Dioxide over Pt / ZrO 2 / Al 2 O 3 Catalysts. 511, 498–511 (2001).
110. Schlapbach, L. & Züttel, a. Hydrogen-storage materials for mobile applications. Nature 414, 353–358 (2001).
111. Zalc, J. M., Sokolovskii, V. & Löffler, D. G. Are Noble Metal-Based Water–Gas Shift Catalysts Practical for Automotive Fuel Processing? J. Catal. 206, 169–171 (2002).
112. Ruettinger, W., Ilinich, O. & Farrauto, R. J. A new generation of water gas shift catalysts for fuel cell applications. J. Power Sources 118, 61–65 (2003).
113. Liu, K., Wang, A. & Zhang, T. Recent advances in preferential oxidation of co reaction over platinum group metal catalysts. ACS Catal. 2, 1165–1178 (2012).
114. Ayabe, S. et al. Catalytic Autothermal Reforming of Methane and Propane over Supported Metal Catalysts. Appl. Catal. A Gen. 241, 261–269 (2003).
115. Asoro, M. a, Kovar, D., Shao-Horn, Y., Allard, L. F. & Ferreira, P. J. Coalescence and sintering of Pt nanoparticles: in situ observation by aberration-corrected HAADF STEM. Nanotechnology 21, 025701 (2010).
116. Pierre, D., Deng, W. & Flytzani-Stephanopoulos, M. The importance of strongly bound Pt-CeOx species for the water-gas shift reaction: Catalyst activity and stability evaluation. Top. Catal. 46, 363–373 (2007).
117. Dong, H., Zhao, J., Chen, J., Wu, Y. & Li, B. Recovery of platinum group metals from spent catalysts: A review. Int. J. Miner. Process. 145, 108–113 (2015).
118. Basu, P. in Essential Readings in Light Metals 176–183 (John Wiley & Sons, Inc., 2013). doi:10.1002/9781118647868.ch23
119. Hischier, R., Hellweg, S., Capello, C. & Primas, A. Establishing life cycle inventories of chemicals based on differing data availability. Int. J. Life Cycle Assess. 10, 59–67 (2005).
120. Kim, S. & Overcash, M. Energy in chemical manufacturing processes: Gate-to-gate information for life cycle assessment. J. Chem. Technol. Biotechnol. 78, 995–1005 (2003).
121. US Census Bureau. 2012 Commodity Flow Survey. 327 (2015).
122. Norling, P., Wood-Black, F., Masciangioli, T. Water and Sustainable Development: Opportunities for the Chemical Sciences – A Workshop Report to the Chemical Sciences Roundtable. National Academy of Sciences (2004).
123. Straganz, G. D., Glieder, A., Brecker, L., Ribbons, D. W. & Steiner, W. Acetylacetone-cleaving enzyme Dke1: a novel C–C-bond-cleaving enzyme from Acinetobacter johnsonii. Biochem. J. 369, 573–581 (2003).
124. Maupin, A. Molly, Kenny, F. Joan, Hutson, S. Susan, Lovelace, K. John, Barber, L. Nancy, Linsey, S. K. Estimated Use of Water in the United States in 2010: U.S. Geological Survey Circular 1405. (2014). doi:http://dx.doi.org/10.3133/cir1405
125. Nehdi, M. & Tariq, A. Stabilization of sulphidic mine tailings for prevention of metal release and acid drainage using cementitious materials: A review. J. Environ. Eng. Sci. 6, 423–436 (2007).
126. Survey, U. S. G. 2012 Minerals Yearbook Platinum-Group Metals. (2016).
127. Luengnaruemitchai, A., Osuwan, S. & Gulari, E. Comparative studies of low-temperature water-gas shift reaction over Pt/CeO2, Au/CeO2, and Au/Fe2O3 catalysts. Catal. Commun. 4, 215–221 (2003).
128. Calzavara, Y., Joussot-Dubien, C., Boissonnet, G. & Sarrade, S. Evaluation of biomass gasification in supercritical water process for hydrogen production. Energy Convers. Manag. 46, 615–631 (2005).
129. Schmal, M. Chemical reaction engineering: essentials, exercises and examples. (2014).
130. Liu, X., Ruettinger, W., Xu, X. & Farrauto, R. Deactivation of Pt/CeO2 water-gas shift catalysts due to shutdown/startup modes for fuel cell applications. Appl. Catal. B Environ. 56, 69–75 (2005).
131. Jha, M. K. et al. Hydrometallurgical recovery/recycling of platinum by the leaching of spent catalysts: A review. Hydrometallurgy 133, 22–32 (2013).
132. PE Americas. Life Cycle Impact Assessment of Aluminum Beverage Cans. Final Report. 1–127 (2010).
133. Sauser, B., Emmanuel, J. & Marquez, R. A System Maturity Index for Decision Support in Life Cycle Acquisition. (2007).
134. Agency, I. E. World Energy Outlook 2016 Executive Summary. (2016).
APPENDICES
Appendix A
| Original Dispersion | 20 | ||||||||
| Catalyst | Time (mins) | T (C) | Dispersion (%) | Loss (%/s) | LN(Loss) | 1/T (K-1) | Pre-Exponential | 6.58E-01 | |
| IW | Activation (J/mol*K) | 5.70E+04 | |||||||
| 60 | 600 | 19 | 0.0003 | -8.1887 | 0.00115 | 300C | 4.22E-06 | %/s | |
| 60 | 800 | 16.7 | 0.0009 | -6.9948 | 0.00093 | OBJFUN | 0.00E+00 | ||
| 60 | 1000 | 7.8 | 0.0034 | -5.6873 | 0.00079 | Time to reach 10% | 2.37E+06 | s | |
| 2.74E+01 | days | ||||||||
| Original Dispersion | 40 | ||||||||
| SCFD | 720 | 800 | 27.52 | 0.0003 | -8.1495 | 0.00093 | Pre-Exponential | 5.49E-02 | |
| 720 | 950 | 15.7 | 0.0006 | -7.4831 | 0.00082 | Activation (J/mol*K) | 4.68E+04 | ||
| 720 | 1000 | 11.87 | 0.0007 | -7.3368 | 0.00079 | 300C | 3.00E-06 | %/s | |
| OBJFUN | 1.60E-14 | ||||||||
| Time to reach 10% | 3.34E+06 | s | |||||||
| 3.86E+01 | days |
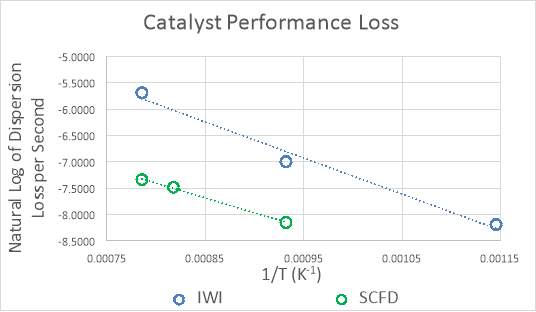
Appendix B
Elemental Analysis Data
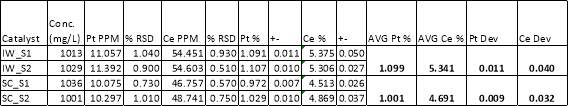
*Digestion procedure insert here*
*Insert AES method here*
Appendix C
Performance Data, GC calibration, Rate Constant Calculations
IW Nanosystem
| No Conversion Ratio | 0.71 | ||||||
| Catalyst Weight | 0.2965 | g | |||||
| Pt Content | 0.003259392 | g | |||||
| Dispersion (fractional) | 0.2 | ||||||
| Exposed Pt | 0.000651878 | g | |||||
| Activation Energy | 74.7528368 | J/mol*K | |||||
| CO Flow (SLPM) | 0.044022 | ||||||
| T ( C ) | Conversion (%) | SD | TOF (s-1) | 1/T | LN(TOF) | Error | Rate (mmol CO/g*Pt/s) |
| 300 | 13.50 | 0.69 | 1.32 | 0.00175 | 0.27459 | 0.05086 | 1.34915 |
| 310 | 19.44 | 3.18 | 1.89 | 0.00172 | 0.63913 | 0.16348 | 1.94258 |
| 320 | 23.77 | 5.60 | 2.32 | 0.00169 | 0.84033 | 0.23541 | 2.37552 |
| 330 | 29.49 | 6.11 | 2.87 | 0.00166 | 1.05599 | 0.20714 | 2.94725 |
| 340 | 38.65 | 2.69 | 3.77 | 0.00163 | 1.32659 | 0.06953 | 3.86313 |
| 350 | 49.86 | 5.03 | 4.86 | 0.00161 | 1.58124 | 0.10082 | 4.98350 |
SCFD Nanosystem
| No Conversion Ratio | 0.71 | ||||||
| Catalyst Weight | 0.2872 | g | |||||
| Pt Content | 0.002873687 | g | |||||
| Dispersion (fractional) | 0.4 | ||||||
| Exposed Pt | 0.001149475 | g | |||||
| Activation Energy | 71.25098 | J/mol*K | |||||
| CO Flow | 0.044022 | SLPM | |||||
| T ( C ) | Conversion | SD | TOF (s-1) | 1/T | LN(TOF) | Error | Rate (mmol CO/g*Pt/s) |
| 300 | 22.25 | 1.53 | 1.23 | 0.00175 | 0.2070 | 0.0687 | 2.5219 |
| 310 | 27.80 | 2.41 | 1.54 | 0.00172 | 0.4300 | 0.0868 | 3.1519 |
| 320 | 34.22 | 2.33 | 1.89 | 0.00169 | 0.6377 | 0.0680 | 3.8794 |
| 330 | 44.24 | 0.37 | 2.45 | 0.00166 | 0.8944 | 0.0083 | 5.0148 |
| 340 | 57.50 | 2.11 | 3.18 | 0.00163 | 1.1565 | 0.0367 | 6.5181 |
| 350 | 73.51 | 8.06 | 4.06 | 0.00161 | 1.4022 | 0.1096 | 8.3334 |
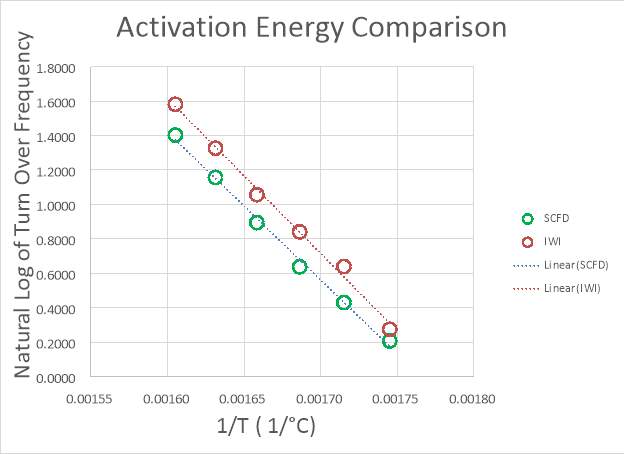
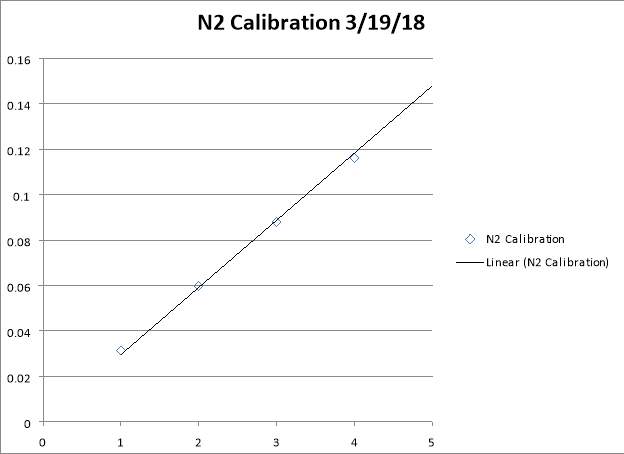
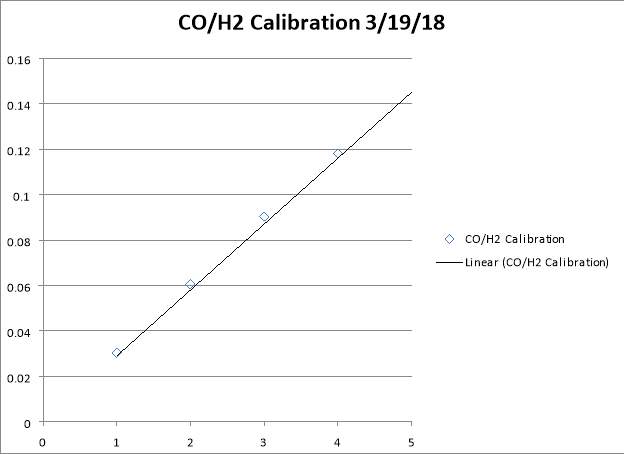
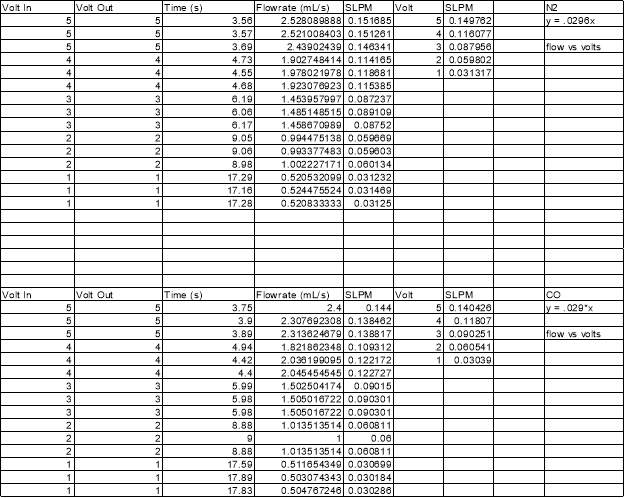
| CO | ||
| Amt Injected (uL) | Area | mol CO |
| 50 | 43054 | 1.41195E-06 |
| 40 | 32127 | 1.12956E-06 |
| 30 | 25801 | 8.4717E-07 |
| 20 | 13913.4 | 5.6478E-07 |
| 10 | 3835 | 2.8239E-07 |
| N2 | ||
| Amt Injected (uL) | Area | mol N2 |
| 50 | 39994.9 | 1.41195E-06 |
| 40 | 33472 | 1.12956E-06 |
| 30 | 22917 | 8.4717E-07 |
| 20 | 16462 | 5.6478E-07 |
| 10 | 6892.7 | 2.8239E-07 |
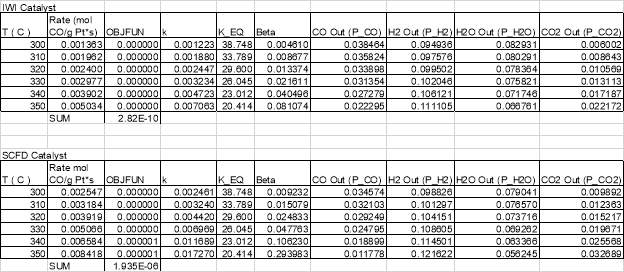
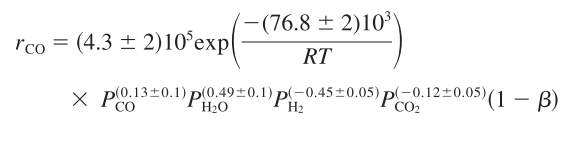
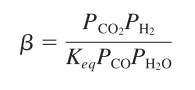
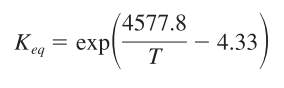
Flow conditions
| N2 Flow (SLPM) | CO Flow (SLPM) | H2 Flow (SLPM) | H2O Flow (SLPM) |
| 0.06 | 0.04 | 0.09 | 0.09 |
Appendix D
Life Cycle Inventory User Defined Materials
Assumptions
- 2.84 grams of water per gram of product122
- 80% of water derived from river source, 20% from well source
- 95% reaction efficiency119
- 99.8% of leftover reactants to water, .2% to air119
- 217 km via truck, 1332 km via rail for all materials121
- Average US energy use and energy source for material production120
| Products | Amount | Notes | |
| Acetylacetone | 10.7139 | g | |
| Avoided products | |||
| Resources | |||
| Water, river, US | 24.3420 | cm3 | |
| Water, well, in ground, US | 6.0855 | cm3 | |
| Materials/fuels | |||
| Isopropenyl Acetate | 11.3136 | g | Thermal arrangement to produce acetylacetone |
| Electricity/heat | |||
| Electricity, medium voltage {US}| market group for | APOS, U | 0.0064 | MJ | |
| Heat, from steam, in chemical industry {RoW}| market for heat, from steam, in chemical industry | APOS, U | 0.0654 | MJ | |
| Heavy fuel oil, burned in refinery furnace {CH}| market for heavy fuel oil, burned in refinery furnace | APOS, U | 0.0016 | MJ | |
| Transport, freight, lorry 16-32 metric ton, EURO3 {GLO}| market for | APOS, U | 0.0023 | tkm | Average distance of 217 km |
| Transport, freight train {US}| market for | APOS, U | 0.0143 | tkm | Average distance of 1332 km |
| Products | Amount | Units | Notes |
| Cerium Chloride | 52.77235 | g | |
| Resources | |||
| Materials/fuels | |||
| Rare earth concentrate, 70% REO, from bastnasite {GLO}| market for | APOS, U | 67.62 | g | |
| Electricity/heat | |||
| Transport, freight, lorry 16-32 metric ton, EURO3 {GLO}| market for | APOS, U | 0.0015 | tkm | Average distance of 217.261 km |
| Transport, freight train {US}| market for | APOS, U | 0.063 | tkm | Average distance of 1332.54 km |
| Products | Amount | Units | Notes |
| Isopropenyl Acetate | 11.31 | g | |
| Avoided products | |||
| Resources | |||
| Water, river, US | 25.69632 | cm3 | |
| Water, well, in ground, US | 6.42408 | cm3 | |
| Materials/fuels | |||
| Acetone, liquid {GLO}| market for | APOS, U | 68.53 | g | |
| Acetic anhydride {RoW}| market for | APOS, U | 60.74 | g | |
| Electricity/heat | |||
| Electricity, medium voltage {US}| market group for | APOS, U | 0.006788136 | MJ | |
| Heat, from steam, in chemical industry {RoW}| market for heat, from steam, in chemical industry | APOS, U | 0.069012716 | MJ | |
| Heavy fuel oil, burned in refinery furnace {CH}| market for heavy fuel oil, burned in refinery furnace | APOS, U | 0.01028016 | MJ | |
| Transport, freight, lorry 16-32 metric ton, EURO3 {GLO}| market for | APOS, U | 0.00246 | tkm | Average distance of 217 km |
| Transport, freight train {US}| market for | APOS, U | 0.0151 | tkm | Average distance of 1332 km |
| Emissions to air | |||
| Acetic anhydride | 0.006393684 | g | |
| Acetone | 0.007213684 | g | |
| Emissions to water | |||
| Acetic anhydride | 3.190448421 | g | |
| Acetone | 3.599628421 | g | |
| Products | Amount | Units | Notes |
| Platinum Chloride (H2PtCl6) | 22.0003 | g | |
| Avoided products | |||
| Resources | |||
| Water, river, US | 49.9846816 | cm3 | |
| Water, well, in ground, US | 12.4961704 | cm3 | |
| Materials/fuels | |||
| Platinum {GLO}| market for | APOS, U | 10.9247 | g | |
| Hydrochloric acid, without water, in 30% solution state {RoW}| market for | APOS, U | 2.04165 | g | |
| Nitric acid, without water, in 50% solution state {GLO}| market for | APOS, U | 14.24026 | g | |
| Electricity/heat | |||
| Electricity, medium voltage {US}| market group for | APOS, U | 0.041800557 | MJ | |
| Heat, from steam, in chemical industry {RoW}| market for heat, from steam, in chemical industry | APOS, U | 0.035200469 | MJ | |
| Heavy fuel oil, burned in refinery furnace {CH}| market for heavy fuel oil, burned in refinery furnace | APOS, U | 0.03300044 | MJ | |
| Transport, freight, lorry 16-32 metric ton, EURO3 {GLO}| market for | APOS, U | 0.00478 | tkm | Average distance of 217.261 km |
| Transport, freight train {US}| market for | APOS, U | 0.0293 | tkm | Average distance of 1332.54 km |
| Emissions to air | |||
| Platinum | 0.001149968 | g | |
| Nitrogen dioxide | 0.001036334 | g | |
| Water | 0.000405818 | g | |
| Emissions to water | |||
| Heavy metals to water (unspecified) | 0.573834242 | g | |
| Nitrogen dioxide | 0.517130876 | g | |
| Water, US | 0.202503125 | cm3 | |
| Products | Amount | Units | Notes |
| Platinum Acetylacetonate | 20.16 | g | |
| Avoided products | |||
| Resources | |||
| Water, river, US | 45.80352 | cm3 | |
| Water, well, in ground, US | 12.58688 | cm3 | |
| Materials/fuels | |||
| Platinum Chloride (H2PtCl6) | 22 | g | |
| Acetylacetone | 10.71391 | g | |
| Water, deionised, from tap water, at user {RoW}| market for water, deionised, from tap water, at user | APOS, U | 93.3191504 | g | |
| Methanol {GLO}| market for | APOS, U | 18.488234 | g | |
| Sodium Acetate | 15.87363705 | g | |
| Electricity/heat | |||
| Electricity, medium voltage {US}| market group for | APOS, U | 0.013200176 | MJ | |
| Heat, from steam, in chemical industry {RoW}| market for heat, from steam, in chemical industry | APOS, U | 0.134201789 | MJ | |
| Heavy fuel oil, burned in refinery furnace {CH}| market for heavy fuel oil, burned in refinery furnace | APOS, U | 0.001607087 | MJ | |
| Transport, freight, lorry 16-32 metric ton, EURO3 {GLO}| market for | APOS, U | 0.00438 | tkm | Average distance of 217.261 km |
| Transport, freight train {US}| market for | APOS, U | 0.0269 | tkm | Average distance of 1332.54 km |
| Emissions to air | |||
| Platinum | 0.00110233 | g | |
| Water | 0.009823068 | g | |
| Methanol | 0.009823068 | g | |
| Chloride | 0.001201911 | g | |
| 2,4-Pentanedione | 0.00112778 | g | |
| Emissions to water | |||
| Heavy metals to water (unspecified) | 0.550062913 | g | |
| Water, US | 4.901711163 | cm3 | |
| Methanol | 4.901711163 | g | |
| Chloride | 0.59975347 | g | |
| 2,4-Pentanedione | 0.56276222 | g | |
| Products | Amount | Units | Notes |
| Sodium Acetate | 15.87363705 | g | |
| Avoided products | |||
| Resources | |||
| Water, river, US | 36.06490338 | cm3 | |
| Water, well, in ground, US | 9.016225844 | cm3 | |
| Materials/fuels | |||
| Soda ash, dense {GLO}| market for | APOS, U | 21.5157264 | g | |
| Acetic acid, without water, in 98% solution state {GLO}| market for | APOS, U | 12.190556 | g | |
| Electricity/heat | |||
| Electricity, medium voltage {US}| market group for | APOS, U | 0.015556164 | MJ | |
| Heat, from steam, in chemical industry {RoW}| market for heat, from steam, in chemical industry | APOS, U | 0.224268146 | MJ | |
| Heavy fuel oil, burned in refinery furnace {CH}| market for heavy fuel oil, burned in refinery furnace | APOS, U | 0.007936819 | MJ | |
| Transport, freight, lorry 16-32 metric ton, EURO3 {GLO}| market for | APOS, U | 0.003448722 | tkm | Average distance of 217.261 km |
| Transport, freight train {US}| market for | APOS, U | 0.021152256 | tkm | Average distance of 1332.54 km |
| Emissions to air | |||
| Acetic acid | 0.012190556 | g | |
| Sodium carbonate | 0.021515726 | g | |
| Emissions to water | |||
| Acetic acid | 6.083087444 | g | |
| Carbonate | 6.11971806 | g | |
| Sodium | 4.616629414 | g | |
| Products | |||
| PtCeOx/Al2O3 via IW (95% Efficiency) | 1 | kg | |
| Avoided products | |||
| Resources | |||
| Water, river, US | 2272 | cm3 | |
| Water, well, in ground, US | 568 | cm3 | |
| Materials/fuels | |||
| Platinum Chloride (H2PtCl6) | 22.1 | g | |
| Cerium Chloride | 52.77235 | g | |
| Aluminium oxide {GLO}| market for | APOS, U | 960 | g | |
| Water, deionised, from tap water, at user {RoW}| market for water, deionised, from tap water, at user | APOS, U | 1152 | g | |
| Electricity/heat | |||
| Heat, from steam, in chemical industry {RoW}| market for heat, from steam, in chemical industry | APOS, U | 0.3 | MJ | Drying of pellets |
| Transport, freight, lorry 16-32 metric ton, EURO3 {GLO}| market for | APOS, U | 0.217 | tkm | |
| Emissions to air | |||
| Platinum | 0.000874695 | g | |
| Water | 0.121263158 | g | |
| Aluminium | 0.053456842 | g | |
| Chloride | 0.002444193 | g | |
| Emissions to water | |||
| Heavy metals to water (unspecified) | 0.436472674 | g | |
| Water, US | 60.51031579 | cm3 | |
| Aluminium | 26.67496421 | g | |
| Chloride | 1.219652333 | g | |
| Products | Amount | Units | Notes |
| PtCeOx/Al2O3 from SCFD (95% efficiency) | 1 | kg | |
| Avoided products | |||
| Resources | |||
| Water, river, US | 2272 | cm3 | |
| Water, well, in ground, US | 2272 | cm3 | |
| Materials/fuels | |||
| Platinum Acetylacetonate | 21.3 | g | |
| Cerium Chloride | 52.77235 | g | |
| Aluminium oxide {GLO}| market for | APOS, U | 960 | g | |
| Water, ultrapure {GLO}| market for | APOS, U | 1152 | g | Incipient wetness + additional water for pellet mixing |
| Electricity/heat | |||
| Heat, from steam, in chemical industry {RoW}| market for heat, from steam, in chemical industry | APOS, U | 65 | MJ | Steam energy to achieve supercritical conditions |
| Transport, freight, lorry 16-32 metric ton, EURO3 {GLO}| market for | APOS, U | 0.217 | tkm | |
| Heat, from steam, in chemical industry {RoW}| market for heat, from steam, in chemical industry | APOS, U | 0.3 | MJ | Drying of pellets after cerium salt addition |
| Emissions to air | |||
| 2,4-Pentanedione | 10.160 | g | |
| Chloride | 0.002 | g | |
| Water | 0.121 | g | |
| Aluminium | 0.053 | g | |
| Platinum | 0.001 | g | |
| Emissions to water | |||
| Chloride | 1.220 | g | |
| Aluminium | 26.675 | g | |
| Water, US | 60.510 | cm3 | |
| Heavy metals to water (unspecified) | 0.554 | g |
Appendix E
Calculations for synthesis phase life cycle inventory
Solubility of platinum precursor at 333 K, 14 MPa: .0000122
| State | T (K) | P (MPa) | Density (kg/m^3) | Enthalpy (kJ/kg) | Entropy (kJ/kg) |
| 1 | 298 | 6.4 | 238.66 | 395.86 | 1.6551 |
| 2 | 333 | 14 | 563.09 | 354.8 | 1.4648 |
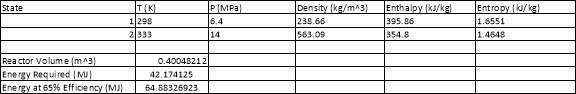
| Compound | Molar mass (g/mol) | Metal fraction | Amount required for 1% Pt, 3% Ce (g) |
| Platinum acetylacetonate | 393.29 | 0.496030919 | 20.16003363 |
| Cerium (III) chloride heptahydrate | 114.116 | 0.306285898 | 97.94770234 |
| Carbon dioxide | 44.01 | N/A | N/A |
Calculations for autothermal reforming of methane coupled with water-gas shift reactor
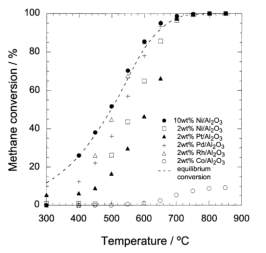 Calculations based on data provided by Ayabe et al.114
Calculations based on data provided by Ayabe et al.114
Pressure = 1 atm
Temperature = 750 °C
Catalyst: 10%Ni/Al2O3
Catalyst density: .56 g/cm3
Reactor volume: 1 cm
Autothermal reforming of methane
CH4+x2O2+1-xH2O↔CO+3-xH2
Steam to methane ratio: 2.5
Oxygen to methane ratio: .1
Reactor temperature: 750 °C
H2/(CO+CO2) Ratio: 3.2
SV: 7200 h-1
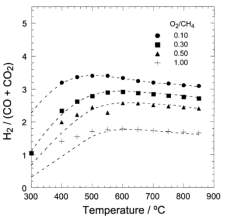
| Reformer | Water-Gas Shift Reactor | |||
| In | Out | In | Out | |
| COMPOUND | Vol % | Vol % | Vol % | Vol % |
| CH4 | 16.7 | 0.0 | 0.0 | 0.0 |
| H2O | 41.8 | 21.8 | 21.8 | 10.9 |
| CO | 0.0 | 7.0 | 7.0 | 3.5** |
| CO2 | 0.0 | 4.8 | 4.8 | 8.3 |
| H2 | 0.0 | 35.8 | 35.8 | 46.7 |
| O2 | 1.7 | 0.0 | 0.0 | 0.0 |
| N2 | 39.9 | 30.6 | 30.6 | 30.6 |
| TOTAL | 100.0 | 100.0 | 100.0 | 100.0 |
* Assumes 100% conversion of methane, 50% conversion of carbon monoxide
** Remaining carbon monoxide assumed to reach acceptable levels during preferential oxidation
Calculations for required hydrogen output for a hydrogen fuel cell
Fuel cell power output: 50 kW
Fuel cell efficiency (LHV): 50 %
Assume STP: 0 °C, 1 atm
Lower heating value (LHV) for
H2+ 12O2→ H2O: 241.82 kJ/mol
ηfuel cell, LHV= PowerH2, flow*LHV
Required hydrogen feed: ≈ .835 g/s or .413 mol/s or .00015 g/H2*s*g WGSR catalyst
Appendix F
*insert published work*
BIOGRAPHICAL SKETCH
Name of Author: Jacob William Deal
Place of Birth: Portsmouth, Virginia
Date of Birth: February 5, 1988
Graduate and Undergraduate Schools Attended: The University of South Alabama
Degrees Awarded:
Bachelor of Science in Chemical Engineering
Master of Science in Chemical Engineering
Awards and Honors:
Alabama Space Grant Consortium Fellowship 2017-2018
Alabama Space Grant Consortium Fellowship 2016-2017
Systems Engineering Doctoral Fellowship 2014-2015
University of South Alabama Three Minute Thesis Winner 2014
Conference of Southern Graduate Schools Three Minute Thesis Finalist 2015
Chemical Engineering Graduate Assistantship 2012-2014
Publications:
Presentation: Deal, J.; Wheeler-West, C.; West, K. (2016). Highly Dispersed Metals on Metal Oxide Supports Via Reactive Deposition from Supercritical CO2. American Institute of Chemical Engineers (AIChE) Annual Meeting. 2016.
Deal, J.; Le, P.; Corey, C.; More, K.; Wheeler-West, C.: Water-Gas Shift Reaction on Alumina-Supported Pt-CeOx Catalysts Prepared by Supercritical Fluid Deposition. Journal of Supercritical Fluids. 2016.
Poster: Deal, J.; Wheeler-West, C.; Corey, B.; Le, P. (2014). Dispersed Pt/Ceria Catalysts for Water-Gas Shift Prepared Using Supercritical Fluid Deposition. American Institute of Chemical Engineers (AIChE) Annual Meeting. 2014.
Castillo, C.; Crepps, G.; Deal, J.; Hellmich, J.; Moore, G.; Nguyen, K. “Now Streaming: A Look into Coastal Mississippi Streams to Map Watershed Boundaries and Model Wetland Extent”. Earthzine. 23 Nov. 2014. Web. 25 Feb 2016.
Presentation: Wheeler-West, C.; Deal, J.; Corey, B.; Le, P. (2014). Water-Gas Shift Reaction On Catalysts Prepared Using Supercritical Fluid Deposition. American Institute of Chemical Engineers (AIChE) Annual Meeting. 2014.
Poster: Wheeler-West, C.; Corey, B.; Deal, J. (2013). Supercritical Fluid Deposition of Dispersed Platinum On Supported Ceria. American Institute of Chemical Engineers (AIChE) Annual Meeting. 2013.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Organic Chemistry"
Organic chemistry is a branch of chemistry that studies the chemical composition, properties, and reactions of organic compounds that contain carbon.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




