Removal of Lead (II) Ions by Adsorption from Biomass
Info: 8563 words (34 pages) Dissertation
Published: 9th Dec 2019
Tagged: ChemistryOrganic Chemistry
ABSTRACT
Environmental pollution by toxic heavy metal has become a challenging problem for maintaining the quality and hygiene of water. The discharges of industrial effluents into Aquatic environment cause a threat for human health. Therefore a batch adsorption Methodology is developed for the removal of toxic metals from aqueous solution. In the present work banana peel was used as adsorbent. Industries in Pakistan are producing wastewater that contains toxic pollutants and pathogens and this issue has been of keen interest to researchers today. Keeping this issue in mind, I have worked on the removal of metallic ions by adsorption. The adsorbent used for this purpose is activated carbon produced from banana peels. It has successfully removed metallic ions from the water under different conditions.
Contents
Introduction………………………………………………………………………………………………………………………………………8
1.2 Standards by World Health Organization
1.3 Pollutants present in Water
1.4.1 Biomass resource of cellulosic banana plant
1.4.2 General uses of banana residues
1.4.3 Utilization of banana pith for waste water treatment
1.4.4 Physio-chemical and structural characteristics of banana fibre
1.4.7 Ash and Moisture Content
Literature Review…………………………………………………………………………………………………………………………….18
Material and Method……………………………………………………………………………………………………………………..21
3.3 Chemical Oxidation and Advanced Oxidation
Results and Discussion…………………………………………………………………………………………………………………….29
4.1 Removal of heavy metals by banana peels
4.2 Effect of Adsorbent Concentration
4.2.1 Graphical Representation
4.3 Effect of time on removal efficiency
4.3.1.1 Graphical Representation
4.3.2.1 Graphical Representation
4.3.3.1 Graphical Representation
4.4 Generalized Concentration vs Time Trend
Conclusion………………………………………………………………………………………………………………………………..……..34
5.1.1 Effects of time on adsorption process
5.1.3 Effect of adsorbent concentration on adsorption
Introduction
1.1 Water
Heavy metals are five times more specific than water. We can define the gravimetric because it is a measure of the thickness of a given number of solids, compared to an equal measure of water. Water is one of the most important things that life sustains on Earth. On our planet, there is 75% water. Ninety-seven percent of the Earth’s water comes in the form of salt, a form of oceans. “And about 3% of the total amount of water is calculated as fresh water in the form of ice sheets, lakes, rivers, groundwater and surface water.” Only one percent is water and the water is always available. Water performs many different important functions in the human body, which are necessary for survival and proper growth.
In some cases heavy metal trace elements. These heavy metals can undoubtedly be identified because this very specific scientific method helps to understand and recognize the metal content with a high degree of accuracy.
Being a major component of cellular water helps cells to function properly.
1. Our body needs energy to work accurately. We derive energy from the different types of food we eat. In fact, food is broken down in water to produce energy.
 2. It also helps to regulate the temperature of the body at high temperatures, keeping the water warm by evaporation.
2. It also helps to regulate the temperature of the body at high temperatures, keeping the water warm by evaporation.
Figure 1. 1 Water Functions in human body
As the world’s population increases, the demand for water is also rapidly increasing. “Water is essential, a healthy life and a pleasing amount of drinking water should be provided to all human beings.” The human body needs different minerals to grow properly and function effectively in small quantities. These minerals include sodium, calcium, magnesium, potassium, chloride. Fluoride and Sulfate The World Health Organization (WHO) has developed drinking water standards to maintain human health.
1.2 Standards by World Health Organization
| Physical Property | Standard |
| Color | ≤15TCU |
| Taste | Non objectionable/Acceptable |
| Odour | Non objectionable/Acceptable |
| Turbidity | <5NTU |
| Total hardness as CaCO3 | <500mg/l |
| TDS | <1000ppm |
| pH | 6.5-8.5 |
| Chemical Properties | Standard |
| Essential Inorganic | Mg/L |
| Aluminum | 0.2 |
| Antimony | 0.02 |
| Arsenic | 0.01 |
| Barium | 0.7 |
| Boron | 0.3 |
| Cadmium | 0.003 |
| Chloride | 250 |
| Chromium | 0.05 |
| Copper | 2 |
| Toxic Inorganic | |
| Cyanide | 0.07 |
| Fluoride | 1.5 |
| Lead | 0.01 |
| Manganese | 0.5 |
| Mercury | 0.001 |
| Nickel | 0.02 |
| Nitrate | 50 |
| Nitrite | 3 |
| Selenium | 0.01 |
| Residual Chlorine | — |
| Zinc | 3 |
Freshwater is polluted and dangerous to humankind due to improper disposal of municipal and industrial waste. It is estimated that by 2025, 66% of the world’s population may live in countries with serious or severe water shortages. Many types of impurities found in drinking-water are dangerous to humans and aquatic organisms, which is why water pollution is the most important reason why living things around the world continue to exist.
Speaking Pakistan in particular, Pakistan ranks 80th out of 122 countries, of which the quality of drinking water shows the poor quality of drinking water in Pakistan. The harmful substances present in the water are considered as water pollutants and are divided into different groups.
1.3 Pollutants present in Water
- Pathogens (bacteria, viruses, protozoa)
- Inorganic pollutants (toxic metals, acids, salts)
- Anions and cations (nitrate, phosphate, Ca + 2, Mg + 2, F–)
- Radioactive material (water-soluble)
In Pakistan, pathogens and toxic metals are the major water pollutants. When it comes to metals, since water is a universal adsorbent, some traces of these metals are naturally added to the water, and trace amounts of these metals are needed in our bodies. If these substances increase or decrease in quantity, they can cause serious damage to the human body and cause many diseases. Most diseases are due to the use of contaminated water.
1.3.1 Toxic metal
Zinc and copper are important for normal human growth. Lack of zinc leads to immune dysfunction, growth failure, abnormalities, neurobehavioral development. According to WHO standards; the allowable limits for copper and zinc are 2 mg / L and 3 mg / L, respectively. In Pakistan, zinc and copper are below WHO standards.
Manganese (Mn) is usually found naturally in fresh water. WHO mainly allows 0.5mg / L limit Mn. In Pakistan, manganese is added to surface water through different sources, which is why magnesium is more abundant in water. It is reported that the highest concentration of manganese found in Pakistan is 2.56 mg / L. The increase in the number of manganese to the WHO limits in surface water and groundwater, at a safety margin.
Iron (Fe) is the most important mineral necessary for the body. Iron is the major component of hemoglobin present in the blood. The body needs the best amount of iron, iron over or under may lead to several problems. In Pakistani iron ore, it exists in groundwater and surface water. According to the World Health Organization, the allowable limit of iron is 0.3mg / L. According to the PCRWR report, groundwater has an iron content of 0-3.7 mg / L and a surface water content of 0.01-9.0 mg / L. Excess iron can cause serious health problems such as cancer, diabetes, liver and heart disease.
Cadmium (Cd) Toxic metals cause serious health damage to the human body. The allowable limit of cadmium for the WHO is 0.003 mg / L. However, in Pakistan, groundwater and surface water have higher levels of cadmium than WHO standards. Exposure to cadmium may result in death cause.
Chromium (Cr) is common in crust and water. Chromium is not a toxic metal and plays an important role in human growth, but its hexavalent state compounds cause excretion, respiration, reproduction, cancer and skin diseases. According to data from different regions, the WHO acceptance of chromium is 0.05 mg / l. Pakistan’s chromium. The report of the Kasul district showed a chromium content higher than the standard value of 2.12 mg / L. Due
to the high chromium content in water, leather and tannery, Lahore, Gujarat, Sialkot and Gujranwala.
Nickel (Ni) metal is found in air, water and ground. The allowable concentration limit for nickel metal given by the World Health Organization is 0.02mg / L. In different cities in Pakistan, nickel concentration varies by region. Groundwater 0-0.366mg / L, surface water 0-1.52mg / L. In Gujranwala, one of the major sources of nickel in surface water is the improper treatment of electroplating industrial effluents. Excess nickel in food and water causes various diseases; respiratory cancer, kidney problems and pulmonary fibrosis.
Lead (Pb) is a common metal in the earth’s crust. Its traces are naturally found in water and soil. The World Health Organization allows the lead content of 0.01mg / L. Potable water can lead to lead contaminants from different sources, such as household coatings, automotive exhaust and the most common industrial emissions. The lead content in China is 0.001 mg / L to 2.0 mg / L in groundwater and 0-0.38 mg / L in surface water. Lead was found to be excessive in water samples from different cities such as Sialkot, Karachi and Khyber Pakthoonkhwa. Excess lead exposure leads to different diseases as follows: Adverse effects on our body systems and organs such as kidneys, digestive system, nervous system, hematopoietic system, cardiovascular system and reproductive system. Even low levels of lead during pregnancy can lead to stunting, underweight and fetus abortion.
Mercury (Hg) is a naturally occurring metal called “persistent biotoxin.” According to WHO standards; Mercury in drinking water does not exceed 0.001 mg / l. Due to the limited date, little research has been done on mercury. According to available information, mercury concentrations in groundwater are within their control limits and comply with WHO standards, but surface water does not meet the given standards. According to one report, samples of Tarbela, Chashma and Lloyd have been quantified by mercury with results of 0.014 mg / L, 0.017 mg / L and 0.14 mg / L, respectively, making it clear that surface water is unsafe for humans. Mercury adversely affects human health and the environment. Mercury has become its toxic compound in aquatic environments, known as methylmercury. Mercury affects the function of nerve cells, disrupts the production of neurotransmitters and reduces the production of thyroid hormones and testosterone in the body.
Arsenic (As) metal is the most dangerous metal in mankind. WHO allows the concentration of 10ppb or 10μg / L. Pakistan’s water concentration has been increasing since the 1990s. According to a 1990 report, Tabula, Chachima, and Lloyds have arsenic concentrations of 620 μg / L, 750 μg / L and 620 μg / L, respectively (8). According to another report The average arsenic concentration in Karachi was 80 μg / L. PCRWR carried out a study of arsenic concentrations found in different parts of Pakistan. As a result of this study, it is clear that arsenic in the areas of Attok and Rawalpindi exceeded their standards. Specifically, the arsenic concentration in the city of Multan is at an astonishing level. Arsenic levels are also high in Sindh, Tai Po and Ganba areas. In some cities of Sindh, the arsenic content exceeds 200 μg / L
Prolonged exposure to arsenic may result in melanosis, leukocytosis, hyperkeratosis, cardiovascular disease, black-foot disease, neuropathy and cancer.
It is clear that all the toxic metals in different parts of Pakistan have different concentrations. All metals of zinc and copper are expected to be over-concentrated and do not meet WHO standards. The PCRWR must take solid action to provide quality water to the Pakistani population. We must stop dealing with municipal and industrial waste.
1.3.2 Anions and Cations
Different anions and cations exist in compounds such as sodium Na +, potassium K +, calcium Ca + 2, manganese Mg + 2 and nitrate NO3-, nitrite NO2-, lead PO-24, carbonate CO3-2, Salt HCO-, Sulfate SO4-2, Chloride Cl- and Fluoride F-. Each ion has a different nature and impact on human health. Some ions naturally occur in water and some ions are caused by improper human activities and waste disposal.
1.3.2.1 Anion
As mentioned above, different types of anions exist in the water. Each anion has its own characteristics and impact on human health. Some are necessary for human growth and health. We get nitrates from different sources, such as food and water. The main source of human nitrate is water. Nitrates are the best amount the body needs, and excessive nitrate can cause health damage and disease. Fertilizer crops such as urea and so on. Due to leaching of farmland, nitrate is excessive. Different studies were conducted in Pakistan, with assessments of different cities in Islamabad, Rawalpindi, Kasur, Lahore, Quetta and Faisalabad and found that nearly 19% of the samples contained excess nitrate. The levels of nitrate in Punjab and Sindh were too high. Nitrate concentrations vary by region and during the weather season nitrate concentrations exceed those in the water during the growing season when fertilizers are applied to crops to ensure their healthy growth. Fertilizers, livestock, manure, and atmospheric sources also increase nitrate concentrations. Excess nitrate may cause the following diseases:
- Methemoglobinemia (blue infant syndrome)
- Respiratory tract infection
- Goiter development (in children)
- Bladder and ovarian cancer
- Genotoxic effects at chromosomal level
Fluoride (F-) is another important anion responsible for the health of teeth. The optimum amount of fluoride is mainly used in the human body. Excess and lack of fluoride can seriously damage human health. The allowable concentration range is 0.7-1.2 mg / L. This concentration is necessary for the prevention of dental caries, but fluoride and spondyloskeletal in over 1.5 mg / L. WHO fluoride concentration standards; in Pakistan, most cities have fluoride levels below 0.7 mg / l in water and more than 1.5 mg fluoride levels in Karachi, Naranji (Khyber Pakhtunkhwa) / L), Nagar Parkar Town and Khalawala, etc. The main source of fluoride ions is the burning of minerals containing fluoride, industrial waste, fertilizers and coal which release fluoride in the air and are subsequently added to the water. The government should adopt a strategy to overcome fluoride deficiency and help to reduce excess fluoride in the water, both of which can cause serious harm to the human body.
Chloride (Cl-1) is mainly found in salt water and is mainly bound to Na +. Phosphate ions are far below the WHO standard limits. In some areas, Pakistani sulphate was found to be excessive. It is near industrial areas because sulfuric acid is the main acid used in different production industries and is the main source of sulfate ions in water due to improper handling of industrial wastewaters.
1.3.2.2 Cation
Each ion is mandatory for normal human growth and body function. The human body needs trace amounts of these cations. Excessive amounts of these cations can cause health injuries. There are many different types of cations in water, such as sodium Na +, potassium K +, calcium Ca + 2, manganese Mg + 2. Sodium (Na +) ions help regulate many of the body’s functions, some of which are as follows:
- To stimulate the circulation of blood and body fluids.
- Nerve impulse function
- Heart function is normal
- Metabolic function
The sodium content varies across Pakistan. It is expected from different studies that the concentration of sodium in flowing water such as rivers and canals is low, but the concentration of sodium in groundwater and lakes is very high because industrial wastewater is allowed to be 0.002 g / L according to WHO Na + ion standard. The PCRWR conducted a study of 23 major Pakistani cities and found that surface and groundwater respectively exceeded 5% and 9% of the WHO standard. Higher Na+ concentration may lead to the following diseases:
- High blood pressure
- hypertension
The human body also needs K +. WHO does not define any potassium standard, but the EU defines a concentration of 12 mg / L of K + ions. Pakistan’s potassium concentration is usually higher than the defined standard.
It is mainly an integral part of our bones and teeth. Who on the Ca + 2 standard is 100mg / L, should not exceed this limit. The groundwater contains too much calcium, compared to surface water, due to the presence of calcareous rocks underground. Prolonged exposure to excess calcium does not affect the body, but prolonged exposure increases blood calcium levels and causes the following:
- Hypercalciuria
- Urinary tract stones
- Calcification in soft tissue
- Inhibit bone remodeling.
The WHO standard for magnesium is 150 mg / l. It is found in controlled concentrations, but in some cities it is one; it is also found to exceed WHO limits.
1.4 Banana Peels
The most important residue is equivalent to 30-40% (weight / weight) with production of compost, proteins, animals, methane and enzymes. The banana peel has a great absorption capacity for metals and many other organic compounds, mainly hydroxyl and carboxyl. 1 gram of banana peel absorbs 5.71 milligrams of cadmium and 2.18 milligrams of lead. Some of the most abundant resources, such as biological macromolecules, are now emerging rapidly and sustainably, at the highest cost and as environmental agencies in many industrial applications. These biological macromolecules are rich in banana fiber resources, of which the potential use of biological resources is the most extensive. Banana fibers are phenolic compounds and the banana peel has adsorption capacity (689 mg / g). With only 2 hours of contact time, the adsorption process is very fast. Desorption experiments show that there is an interaction of chemical adsorption between natural phenol sites and banana peel adsorption.
the production / production of bananas tons. bananas, banana plantations, banana production, banana production. 11% of the world surface of bananas in Brazil, Tanzania, the Philippines and China. The four main banana exporters. Because banana plantations increase and cause great concern, they are considered waste and are used in the field.
Literature Review
The most plenteous compound on the earth is a water which is very important for the survival for any single living being. Around half of the population which is mainly rely on the ground of the water. Ground water is used in many areas of Pakistan as a drinking purpose. we know that essential requirement of a human advancement and health is a safe drinking water,so with that human right can be globally acknowledged.
Supply of safe, clean and plenteous water for drinking reasons for existence is really important for a good health. United nations indicating a report that about to be 884 million individuals are not getting a clean water. The main problem for the for a international community is a availability of a drinking water. With aspects of a population increases and water contamination, availability of a safe drinking water is turned into a worldwide testing into a devolving nations. Water contamination in a earth has a critical problem for a earth and also for a human health.it is also come to know that a large number of individuals from a poor and under devolved countries kicked our the buckets every year subsequently of the diseases which are preventable diseases.
Population is increasing day by day and step by step and that consistent increment in population brings about deficiency of fresh water here. The population which constantly lift the necessity of water for the generation of stuff like food , horticulture , industry and household usage.in our country Pakistan , water is going to be contaminated because of municipal and untreated water and also from the industrial and agriculture waste, As per four years water think about led by Pakistan council of research in water resources during 2002-2006that about to 84-89% of the water source in the nations are be contaminated
Numerous nations are mainly facing modern industrial exercises for a waste water treatment and heavy metals , particularly devolving nations. Heavy metals like arsenic, nickel, cadmium, lead when releases into water of rivers and without any good treatment whose outcome is a extreme contamination , promoting natural effects on amphibian life of plants and also of the ecosystem. Numerous heavy metals are manily accumulated biologically and also not possible integrated , imperilling human health Many tyoes of industrial waste water mainly containing the incorporate complex of organic compounds , mines, electric power plants , electroplating and quarries.
Past researches shows when the channel, tube well, streams and other municipal water test were assessed as a main source of heavy metals in various regions of Punjab like Gujranwala and Shekhupura. Mainly arsenic , lead , nickel and chromium are identified .over the specific level by WHO. Mainly it is observed that hand umps and tube wells have much abnormal state of arsenic than that of the other streams.
Eliminate the heavy water is very important because these are not only harmful for the aquatic life but also for the human being. These heavu metals not only destroy our system but also can leads towards human death if it becomes a very serious. It is mainly found in a drinking water so that’s why we have to work on it.
WHO and EPA makes some standards which we all have to follow for a healthy life which can safe to us from these heavy metals water and we can easily get a good drinking water. There are many methods used for a drinking water. Some of a methods are very expensive and also a time requiring.
There are many methods which are created fot the water purifications such as a electrochemical procedure, membrane filtration, coagulation, oxidation process by Szpyrkowics, adsorption by nagah and hanafiah 2008 and also the one of the most important technique now a days which is knows as a reverse osmosis for the treatment of various sources of water. Among these techniques which I used for that is a adsorption technique no matter it is slightly costly but devision procedure for the elimination of metal particles from contamined water,
The main purpose of the thesis is that on which I have work is the utilizing a low cost method for the eliminations of the heavy metals from the drinking water. Adsorption method is usually a method from which heavy metals can be removed froma drinking water. Many adsorbents are efficient which likw silica, grapheme, activated carbon is usually used. Most the adsorbents which are like activated carbon which is mostly used for the removal of heavy metals the reason is that activated carbon provides a much large surface area whose range is about from 500- 1500 meter square per gram but it is also so expensive to use for the elimination of heavy metals from water industrial waste can be uised. These adsorbent are not only economical but they also helps to us for the removal of heavy metals.
In my work the research adsorbent like banana peels are used for the removal of heavy metals specially like Lead which I am going to remove also chromium, arsenic and cadmium from the water. The banana peel has a high adsorption capacity for metals and organic compounds. The main reason for this is the presence of hydroxyl and carboxyl groups in the pectin. The adsorption of lead on banana peel has been studied in batch mode. The maximum adsorption of banana peel by the Langmuir isotherm indicates that 1 gram of banana peel adsorbs 2.18 mg of lead.
Material and Methods
Heavy metal contaminants are present in fluid waste in many industries, such as mining operations, metal plating, tanning plants, refining, radiator manufacturing, smelting and battery operations. Forms of treatment for removal of heavy metals from drinking water include precipitation, exchange, membrane filtration, co precipitation and adsorption. The study found that the treatment of heavy metals in bearings has shown that adsorption is a profound and potent strategy for removing heavy metals from the waste stream.
There are usually many ways to remove heavy metals from drinking water. In this chapter, we discuss different ways to remove heavy metals from drinking water and then choose the appropriate and economical way to remove heavy metals from drinking water.
3.1 Adsorption
Activated carbon can be castoff to confiscate organic contents of water that react with disinfectants and yield DBPs. This is, contrariwise, affluent, while biological activated carbon (BAC), which often encompasses ozone and granular activated carbon (GAC), may be more cost-effective in some cases.
Fundamentally, adsorption is through the process of mass transfer in which matter is exchanged from a fluid to a solid surface, and significantly incorporates additional chemical and physical interactions. Today, adsorption has become one of the strategic options for treating water pollution by removing heavy metals (Kurniawan and Babel, 2003)
Recently, the economic and security strategies required to remove heavy metals from wastewater also need to be studied. Articles Low cost agricultural waste, such as bagasse, rice husks, seashells, and fly ash from banana peel Heavy metal removal Many analysts have explored water, Topic: Cost is a must obey these adsorbent parameters. In any case, the data rarely shows that the cost of a single adsorbent fluctuates depending on the level of management and accessibility required. In fact, the adsorbent can be named as the minimum cost of the adsorbent in the case that it requires a small amount of handling as a by-product of other industries. Obviously, the improved adsorption limit can offset the cost of additional processing.
3.2 Membrane Technology
Advances in membranes, used archaeologically to desalinate salty waters, endure to demonstrate tremendous confiscation of natural organic matter. Membrane practices use hydraulic pressure to force water through a quasi-penetrable membrane that discards most contaminants. The disparities of this technology embrace reverse osmosis, Nano filtration (low pressure reverse osmosis), ultrafiltration, microfiltration and (similar to the filtration of conformist sand).
The further elimination of DBP control strategies involves varying the chlorination point for later in the treatment process they have achieved challenge with updated PAD protocols with the help of one or more of these progressions. Water system directors can also deliberately convert chlorine to one or more marginal disinfectants to decrease the materialization of THM and HAA. However, all disinfectants result in some disinfection byproducts, many of which persist mysteriously, while related DBP groups (eg, nitrogenous-DBP) endure to be identified means that is known about splits of substitutions to chlorination that is notorious about DBP chlorination much less. In addition, disinfection individually has rewards and obstacles.
3.2.1 Advantage
- Excessively effective in contradiction with bacterial and viral aquatic pathogens and some protozoa.
- Provides an excellent level of disinfectant to help coat next to a new microbial growth and decrease the progression of the biofilm in the diffusion system.
- No pragmatic, precise and scrutinized effort.
- Operationally simple and with maximum firmness.
- The most lucrative disinfectant.
3.3 Chemical Oxidation and Advanced Oxidation
A chemical strategy to expel heavy metals from wastewater, another technique that is based on liquid crystal glass is now generally used. Chemical oxidation includes the knowledge of an oxidizing agent in water, causing the electrons to move from the oxidant to the pollutants, which undergo a basic adjustment of the structure and become less dangerous. In any case, chemical oxidation can lead to contaminated products. Oxidation processes, for example, air extraction, vaporization and adsorption by activated carbon can help eliminate any contaminated product. Advanced oxidation and chemical oxidation are constantly used for the pre-treatment of drinking water of heavy metals containing organic and inorganic compounds.
3.4 Chemical Precipitation
The precipitation of substances includes the addition of a precipitation reagent to contaminated water, which causes chemical changes that convert metals into strong particles. The particles can accumulate by coagulation and evacuate by filtration. It is the most widely recognized technique that expels dissolvable heavy metals from contaminated water. It is the not expensive method for the removal of heavy metals from contaminated water. In any case, it is not efficient to treat water contaminated with highly corrosive substances. Provides a large amount of contaminated dirt that must be treated with chemical adjustment and properly disposed.
3.5 Chemical coagulation
Chemical coagulation continues to use the chemical coagulation of the small and strong contamination of the stained water particles and, therefore, chemical flocculation. The particles typically have a negative charge that prevents the particles from forming deposits and bodies. With a positively charged coagulant, it reduces the charge on the negative particles, which causes them to form very large concentrations. The anionic flocculants familiar with the mixture can react with the positively charged mixture, bind the particles to a high concentration and can be emptied by filtration. In addition to chemical precipitation, coagulation and sedimentation through chemical products is one of the most economical ways to extract heavy metals from contaminated water. It is not corrosive and, therefore, is detrimental to the treatment of highly concentrated corrosive wastewater. Sediments also contain heavy metals that must be treated by other methods, such as dehydration or neutralization.
3.6 Ion exchange
A chemical reaction takes place during ion exchange, in which heavy metal particles from polluted water are exchanged into equally charged particles. The solid particles either normally take the form of inorganic zeolites or organic resins that are usually produced synthetically. The ability to recycle adsorbent metals is a process of ion exchange. However, ion exchange is the most costly method, with more than S3 dollars per ton of wastewater containing 100 ppm of heavy metals. This technique is also limited by the highly corrosive levels of polluted water networks and the high concentration of heavy metals and therefore requires that prior to process pre-treatment.
3.7 Rice Husk
This waste material contains different compositions such as cellulose, 3% crude protein, 25% hemicelluloses, silica (including ash) and lignin. It has 70-85% natural protein. material such as lignin, sugar, cellulose and the rest of silica, which is available in the cell layer.
Lately, the use of the adjusted rice husk has been considered and is used for the expulsion of contaminants from the water since adsorption studies have been carried out on the use of rice husk as an adsorbent for the removal of heavy metals and have the impact of the different parameters, for example, pH, adsorbent concentration, temperature, particle size, etc. It was considered that the rice husk is a conceivably useful material for the removal of heavy metals from water
3.8 Egg Shell
Egg shell waste is the basic product of the egg processing industry. Due to its major production, environmental risks and money-related burdens, the transfer of this waste needs to be very much about its own. In this way, choices should be made to increase the value of modern waste (Russ and meyer-pittroff, 2004). The egg shell is largely a crystallisation of Cacos. Calcium comes from the hen’s bones. Since the high temperature protein in the egg shell can be inactivated above 40 ° C, the normally boiled duck and egg shell were washed several times with distilled water, air-dried and placed in a hot air oven at 40 ° C for 30 minutes. The grinder is ground in powder form and then the powder is used to eliminate heavy metals, which contains 80-90% of 6 Caco, which is very effective in removing heavy metals from water. There is no effective adsorbent for removal of heavy metals from water compared to calcined eggshell
3.9 Fly Ash
Fly ash is the coal burned by the items. It is extracted by a dust collector in the chimney of a coal consumer plant to reduce pollution. Because of the transfer problems that fly ash has been affected by the pollution control framework in the 1960s and 1970s, extensive studies have been conducted to understand its usefulness as a soil stabilizer and a mixture of basic solid structures.
3.10 Banana Peels
Banana peel containing sulphur, nitrogen and some organic compounds such as atoms of carboxylic acids. They are lingo cellulose compounds in natural materials that contain hydroxyl, carboxyl, and ester functional groups. Banana peel has a protein composition of 0.9%,Lipid 1.7%, crude fibre 31% or sugar 59%. The different minerals present are Manganese 76.20 mg / g, Potassium 78. 10 mg / g, 24.30 mg ng / g sodium, 0.61 mg / g iron and 19.20 mg / g calcium. These banana peels washed with purified water several times after washing. Banana peel is a very good product for all adsorbents and can be used as an important material for cleaning water. Biosorbents made from banana peel have been used to eliminate chromium, copper and cadmium in water.
3.11 Activated carbon
Activated carbon is a solid inert adsorbent material usually used to expel different soluble contaminants from the water and process the streams of the gas phase. It is produced using any raw material that contains carbon, including the family of coal and coconut shells, no doubt the amount of persecutors will know.
Adsorption is the accumulation of a fluid or gas on the surface of a solid or liquid substrate, instead of the absorption in which the infringing substance enters the mass or volume of the substrate.
 Activated carbon is permeable, reasonable and readily accessible for use as adsorbents, providing a large surface area to remove contaminants. It has a more useful surface area / gram than any other material accessible for physical adsorption. Actually, the teaspoon of activated carbon has more surface than a football field
Activated carbon is permeable, reasonable and readily accessible for use as adsorbents, providing a large surface area to remove contaminants. It has a more useful surface area / gram than any other material accessible for physical adsorption. Actually, the teaspoon of activated carbon has more surface than a football field
3.12 Material and Chemicals
Very firstly I bought the materials and chemicals from the market required for performing the experimental work. Material like 2 dozen banana peels bought from the local Market Ichara Lahore.
3.13 Banana Peels
I cut the banana peels into pieces and then washed 2 times with distilled water and 2 times with a tap water to expel the diet from it. For removing the moisture from the surface these banana peels were kept in air and then dried in an oven for almost 6 hours at 100 degree Celsius. These banana peels were crushed into a powder form by putting into a hydrogenate.



- Agitator
- Pressure gauge
- Reaction vessel
- Gas inlet
- Gas outlet
- Control panel
Reactor is a tall vertical vessel made up of stainless steel, provided with an external jacketed heating. Reactor vessel having a capacity of 3 liter is also equipped with an agitator driven with an help of electric motor.
Preparation of Heavy metal solution in a distilled water:
For preparation of heavy metal solution in distilled water we have to make the following solution.
Lead Nitrate:
Very firstlyfor making the solution of heavy model we have to make a solution for a 500 ppm solution. For that we have to take a lead nitrate of 0.3996 which we calculated. Just after that we make a solution of 50ppm solution in which I make a 500 ml of sample in which 50ml taje from the above sample and 450ml water added in it. Very next for the 10ppm solution, 20ml sample take from the 500 ppm solution and more 80ml water added in it. For 20ppm solution, 240ml solution take from a 500 ppm standard solution and 360ml water added in it. Finally for a 30ppm solution, we take sample 60 ml from a 500 ppm solution and added 40 ml water in it. With that we make our model solution. Further we use now catalyst lab for using Absorption spectrometer.
Results and Discussion
When samples of water tested in the laboratory by using the atomic adsorption spectrometer. After that we are able to obtain the % removal of heavy metals by comparing to that with an initial amount of the heavy metals. Results are going to be discussed in details.
4.1 Removal of heavy metals by banana peels
Banana peels contain the composition of sulphur, nitrogen and carboxylic acid which are so effective for the removal of heavy metals. Banana peels mainly contain 40% mass of its fruit. That has been proved Banana peels can be reused again as a adsorbent for the removal of heavy metals many times.
When 1 gram of banana peels are utilized for each of the sample of water for the removal of heavy metal by varying the concentration and contact time we obtain the following results.
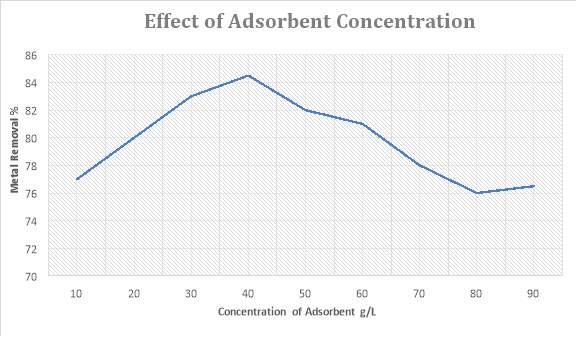
4.2 Effect of Adsorbent Concentration
We have checked the effect of adsorbent concentration on its removal efficiency. For this, I have used 50ppm solution of lead ions as standard solution. I have added 10-90gm of adsorbent in the 100 ml solution of 50ppm separately and made nine sample. Agitation speed for this experiment is 100rpm and left the sample for 30mins to shake.
| Amount of Adsorbent (grams) | Removed Amount mg/L | Metal removal % |
| 10 | 38.5 | 77% |
| 20 | 40 | 80% |
| 30 | 41.5 | 83% |
| 40 | 42.25 | 84.5% |
| 50 | 41 | 82% |
| 60 | 40.5 | 81% |
| 70 | 39 | 78% |
| 80 | 38 | 76% |
| 90 | 38.25 | 76.5% |
The quantification of nine samples revealed that the adsorption is maximum for 40g/L of adsorbent dose. If we increase the adsorbent dose adsorption will become less same is with using adsorbent less than 40g/L. Optimum amount of adsorbent for removal of lead ions is 40g/L.
It is assumed that if we increase the amount of adsorbent, the adsorption will be high due to the presence of increased active sites but results shows divergence from hypothesis.
The reason for this if we increase the amount of adsorbent, it will aggregate and the no of active sites will decrease.
 4.2.1 Graphical Representation
4.2.1 Graphical Representation
4.3 Effect of time on removal efficiency
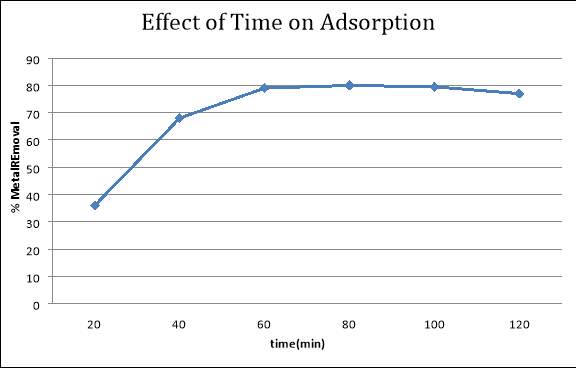
In order to investigate the effect of time on the removal efficiency of lead ions I used 50 ppm solution as standard and took the sample at different intervals of times and quantified those sample using atomic adsorption spectrometer. The results shows that for 80mins the adsorption increases and after 80minss adsorption decreases.
50ppm Standard Solution
| Time(min) | Amount Adsorbed | % Metal Removal |
| 20 | 18 | 36% |
| 40 | 34 | 68% |
| 60 | 39.5 | 79% |
| 80 | 40 | 80% |
| 100 | 39.5 | 79.5% |
| 120 | 38.5 | 77% |
4.3.1 Graphical Representation
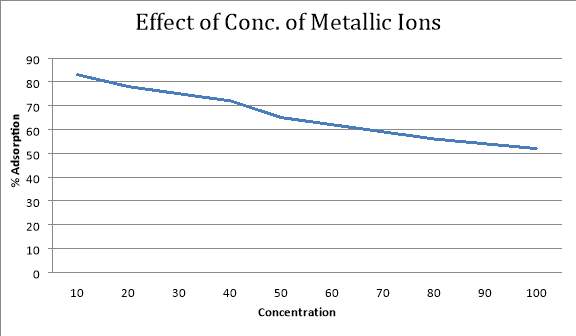
4.4 Effect of metal ion concentration
I have tested the effect metal ions concentration on adsorption by keeping the dose of adsorbent constant. The result shows to us that increasing initial concentration from 10-100mg/L removal efficiency decreases. The adsorption percentage of lead decreases as the initial concentration increased from 10mg/l to 100 mg/L. Maximum adsorption is obtained at the minimum concentration of metallic ions. Such behaviour is due to that because of the unchanging amount of available active sites on the adsorbent because the amount of adsorbent was constant.
| Concentration of Adsorbent(ppm) | % Adsorption | Adsorbed Amount |
| 10 | 83% | 8.3 |
| 20 | 78% | 15.6 |
| 30 | 75% | 22.5 |
| 40 | 72% | 28.8 |
| 50 | 65% | 32.5 |
| 60 | 62% | 37.2 |
| 70 | 59% | 41.3 |
| 80 | 56% | 44.8 |
| 90 | 54% | 48.6 |
| 100 | 52% | 52 |
4.4.1 Graphical Representation
Conclusion
5.1 Overview
This research is aimed to find out the adsorption characteristics of metallic ions present in water by use of the activated carbon produced from banana peel. The outcomes of this research revealed that activated carbon produced from banana peel has outstanding metallic ions adsorption characteristics. The parameters considered in this research work are given below:
- Effects of time on adsorption process
- Effect of adsorbent concentration on adsorption
- Effect of Metallic Ion Concentration
5.1.1 Effects of time on adsorption process
From the results of experimentations it is observed that the optimum time for the adsorption is almost 80min using banana peels.
5.1.3 Effect of adsorbent concentration on adsorption
The optimum amount of adsorbent used for the removal of metallic ions in 40g/L. Beyond this amount the efficiency of the adsorption will decrease.
5.1.4 Effect of Metallic Ion Concentration
If we increase the metallic ion concentration having the dose of adsorbent constant, the adsorption will decrease. This happens because the no of active will reduce if we increase the amount of metallic ions.
References
1. World Health Organization. Guidelines for drinking water quality. Geneva : world health organization, 1993.
2. Water pollution in Pakistan and its impact on public health. Peter Richter. 2011, Environment International, Vol. 37, pp. 479-497.
3. The importance of zinc in human nutrition and estimation of the global prevalence of zinc defi ciency. Kenneth H. Brown.
4. Heavy metals contamination through industrial effluent to irrigation water and soil in Korangi area of Karachi(Pakistan). Midrar-ul-haq. 2005, Int J Agri, Vol. 7, pp. 646-648.
5. PCRWR. Nationa water quality motinoring program. Islamabad, Pakistan : Water Quality Report, 2003-2004.
6. Assessment of groundwater contamination in an industrial city, silakot, Pakistan. Ullah R. 2009, Afr J Environ Sci Technol, Vol. 3, pp. 429-446.
7. Lead toxicity. GIDLOW, D.A. 2, March 1, 2004, Occupational Medicine, Vol. 54, pp. 76-81.
8. Contents of trace metals in fish, sediment andwater from three freshwater reservoirs on theIndus River, Pakistan. M.Ashraf, Jaleel Tariq and M. Jaffar. Amsterdam : s.n., 1991, Elsevier Science Publishers B.V., Vol. 12, pp. 355-364.
9. PCRWR. Annual report. Islamabad : Pakistan Council for Research in Water Resources, 2005-2006.
10. Water pollution in Pakistan and its impact on public health. Muhammad Nasir Khan Khattak. 2011, Environment International, Vol. 37, pp. 479-497.
11. Distribution of nitrate in the water resources of Pakistan. Tahir MA; Rasheed H;. 2008, Afr J Environ Sci Techol, Vol. 2, pp. 397-403.
12. (CDCP), Center for Disease Control and Prevention. fluoridation of drinking water to prevent dental caries. United States : Morb Mortal Wkly Rep, 1993. pp. 933-940.
13. V.K. Gupta, Suhas, Application of low-cost adsorbents for dye removal – a review, J. Environ. Manag. 90 (2009) 2313-2342.
14. National Horticulture Board (NHB) home page Retrieved on 16th Aug 2014. http://nhb.gov.in
15. H.K.Y. Reddy, M. Srijana, M.D. Reddy, G. Reddy, Afr. J. Biotechnol. 9 (2010) 1926–1934
16. R. Roy, B.K. Sarkar, N.R. Bose, Bull. Mater. Sci. 24 (2001) 137–142
17. water by using “bio-adsorbents”. Int. J. Res. Eng. Technol. 3 (4), 776e785.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Organic Chemistry"
Organic chemistry is a branch of chemistry that studies the chemical composition, properties, and reactions of organic compounds that contain carbon.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please: